Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia
- PMID: 32762557
- PMCID: PMC7509272
- DOI: 10.1152/ajpheart.00202.2020
Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia
Abstract
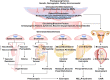
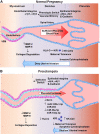
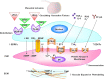
Preeclampsia is a major complication of pregnancy manifested as hypertension and often intrauterine growth restriction, but the underlying pathophysiological mechanisms are unclear. Predisposing genetic and environmental factors cause placental maladaptations leading to defective placentation, apoptosis of invasive cytotrophoblasts, inadequate expansive remodeling of the spiral arteries, reduced uteroplacental perfusion pressure, and placental ischemia. Placental ischemia promotes the release of bioactive factors into the maternal circulation, causing an imbalance between antiangiogenic soluble fms-like tyrosine kinase-1 and soluble endoglin and proangiogenic vascular endothelial growth factor, placental growth factor, and transforming growth factor-β. Placental ischemia also stimulates the release of proinflammatory cytokines, hypoxia-inducible factor, reactive oxygen species, and angiotensin type 1 receptor agonistic autoantibodies. These circulating factors target the vascular endothelium, causing generalized endotheliosis in systemic, renal, cerebral, and hepatic vessels, leading to decreases in endothelium-derived vasodilators such as nitric oxide, prostacyclin, and hyperpolarization factor and increases in vasoconstrictors such as endothelin-1 and thromboxane A2. The bioactive factors also target vascular smooth muscle and enhance the mechanisms of vascular contraction, including cytosolic Ca2+, protein kinase C, and Rho-kinase. The bioactive factors could also target matrix metalloproteinases and the extracellular matrix, causing inadequate vascular remodeling, increased arterial stiffening, and further increases in vascular resistance and hypertension. As therapeutic options are limited, understanding the underlying vascular mechanisms and molecular targets should help design new tools for the detection and management of hypertension in pregnancy and preeclampsia.
Keywords: cytokines; endothelium; hypertension; placental ischemia; vascular smooth muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Akehurst C, Small HY, Sharafetdinova L, Forrest R, Beattie W, Brown CE, Robinson SW, McClure JD, Work LM, Carty DM, McBride MW, Freeman DJ, Delles C. Differential expression of microRNA-206 and its target genes in preeclampsia. J Hypertens 33: 2068–2074, 2015. doi:10.1097/HJH.0000000000000656. - DOI - PMC - PubMed
-
- Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi:10.1161/01.HYP.37.4.1191. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

