High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial
- PMID: 32762701
- PMCID: PMC7407427
- DOI: 10.1186/s13054-020-03214-9
High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial
Abstract
Background: High-flow nasal cannula (HFNC) oxygen therapy is being increasingly used to prevent post-extubation hypoxemic respiratory failure and reintubation. However, evidence to support the use of HFNC in chronic obstructive pulmonary disease (COPD) patients with hypercapnic respiratory failure after extubation is limited. This study was conducted to test if HFNC is non-inferior to non-invasive ventilation (NIV) in preventing post-extubation treatment failure in COPD patients previously intubated for hypercapnic respiratory failure.
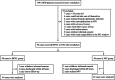
Methods: COPD patients with hypercapnic respiratory failure who were already receiving invasive ventilation were randomized to HFNC or NIV at extubation at two large tertiary academic teaching hospitals. The primary endpoint was treatment failure, defined as either resumption of invasive ventilation or switching to the other study treatment modality (NIV for patients in the NFNC group or vice versa).
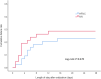
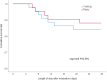
Results: Ninety-six patients were randomly assigned to the HFNC group or NIV group. After secondary exclusion, 44 patients in the HFNC group and 42 patients in the NIV group were included in the analysis. The treatment failure rate in the HFNC group was 22.7% and 28.6% in the NIV group-risk difference of - 5.8% (95% CI, - 23.8-12.4%, p = 0.535), which was significantly lower than the non-inferior margin of 9%. Analysis of the causes of treatment failure showed that treatment intolerance in the HFNC group was significantly lower than that in the NIV group, with a risk difference of - 50.0% (95% CI, - 74.6 to - 12.9%, p = 0.015). One hour after extubation, the mean respiratory rates of both groups were faster than their baseline levels before extubation (p < 0.050). Twenty-four hours after extubation, the respiratory rate of the HFNC group had returned to baseline, but the NIV group was still higher than the baseline. Forty-eight hours after extubation, the respiratory rates of both groups were not significantly different from the baseline. The average number of daily airway care interventions in the NIV group was 7 (5-9.3), which was significantly higher than 6 (4-7) times in the HFNC group (p = 0.006). The comfort score and incidence of nasal and facial skin breakdown of the HFNC group was also significantly better than that of the NIV group [7 (6-8) vs 5 (4-7), P < 0.001] and [0 vs 9.6%, p = 0.027], respectively.
Conclusion: Among COPD patients with severe hypercapnic respiratory failure who received invasive ventilation, the use of HFNC after extubation did not result in increased rates of treatment failure compared with NIV. HFNC also had better tolerance and comfort than NIV.
Trial registration: chictr.org ( ChiCTR1800018530 ). Registered on 22 September 2018, http://www.chictr.org.cn/usercenter.aspx.
Keywords: Chronic obstructive pulmonary diseases; High-flow nasal cannula; Hypercapnia; Non-invasive ventilation; Pulmonary infection control window; Respiratory failure.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Comment in
-
High-flow nasal cannula can't be considered non-inferior to noninvasive ventilation in patients with chronic obstructive pulmonary disease who develop respiratory failure after extubation.Crit Care. 2020 Nov 23;24(1):659. doi: 10.1186/s13054-020-03363-x. Crit Care. 2020. PMID: 33228758 Free PMC article. No abstract available.
References
-
- Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1249–1256. doi: 10.1164/ajrccm.159.4.9807050. - DOI - PubMed
-
- Li PJ, Wang T, Xiao J, Jiang FM, Luo J, Shi CL, Liu GJ, Liang ZA. Efficacy of two noninvasive weaning strategies in intubated patients with chronic obstructive pulmonary disease: a meta-analysis and indirect treatment comparison. Heart Lung. 2016;45(2):132–139. doi: 10.1016/j.hrtlng.2015.12.008. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- R2017003/Rui E special fund for emergency medicine research/International
- YZ2018090/Yangzhou Science and Technology Development Plan/International
- 2018034/Yangzhou Phase III Talent Cultivation Program Support Project/International
- yzucms2018943, fcjs201708, fcjs201842/hospital-level support project of Northern Jiangsu People's Hospital/International
LinkOut - more resources
Full Text Sources
Medical

