Low-Dose Chest CT for the Diagnosis of COVID-19—A Systematic, Prospective Comparison With PCR
- PMID: 32762834
- PMCID: PMC7465363
- DOI: 10.3238/arztebl.2020.0389
Low-Dose Chest CT for the Diagnosis of COVID-19—A Systematic, Prospective Comparison With PCR
Abstract
Background: Only limited evidence has been available to date on the accuracy of systematic low-dose chest computed tomography (LDCT) use in the diagnosis of COVID-19 in patients with non-specific clinical symptoms.
Methods: The COVID-19 Imaging Registry Study Aachen (COVID-19-Bildgebungs-Register Aachen, COBRA) collects data on imaging in patients with COVID-19. Two of the COBRA partner hospitals (RWTH Aachen University Hospital and Dueren Hospital) systematically perform reverse transcriptase polymerase chain reaction (RT-PCR) from nasopharyngeal swabs as well as LDCT in all patients presenting with manifestations that are compatible with COVID-19. In accordance with the COV-RADS protocol, the LDCT scans were prospectively evaluated before the RT-PCR findings were available in order to categorize the likelihood of COVID-19.
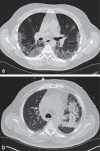
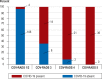
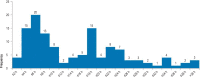
Results: From 18 March to 5 May 2020, 191 patients with COVID-19 manifestations (117 male, age 65 ± 16 years) underwent RT-PCR testing and LDCT. The mean time from the submission of the sample to the availability of the RT-PCR findings was 491 minutes (interquartile range [IQR: 276-1066]), while that from the performance of the CT to the availability of its findings was 9 minutes (IQR: 6-11). A diagnosis of COVID-19 was made in 75/191 patients (39%). The LDCT was positive in 71 of these 75 patients and negative in 106 of the 116 patients without COVID-19, corresponding to 94.7% sensitivity (95% confidence interval [86.9; 98.5]), 91.4% specificity [84.7; 95.8], positive and negative predictive values of 87.7% [78.5; 93.9] and 96.4% [91.1; 98.6], respectively, and an AUC (area under the curve) of 0.959 [0.930; 0.988]. The initial RT-PCR test results were falsely negative in six patients, yielding a sensitivity of 92.0% [83.4; 97.0]; these six patients had positive LDCT findings. 47.4% of the LDCTs that were negative for COVID-19 (55/116) exhibited pathological pulmonary changes, including infiltrates, that were correctly distinguished from SARS-CoV-2 related changes.
Conclusion: In patients with symptoms compatible with COVID-19, LDCT can esablish the diagnosis of COVID-19 with comparable sensitivity to RT-PCR testing. In addition, it offers a high specificity for distinguishing COVID-19 from other diseases associated with the same or similar clinical symptoms. We propose the systematic use of LDCT in addition to RT-PCR testing because it helps correct false-negative RT-PCR results, because its results are available much faster than those of RT-PCRtesting, and because it provides additional diagnostic information useful for treatment planning regardless of the type of the infectious agent.
Figures


Comment in
-
Post-Test Probability of COVID-19 Using CT.Dtsch Arztebl Int. 2021 Feb 5;118(5):66. doi: 10.3238/arztebl.m2021.0036. Dtsch Arztebl Int. 2021. PMID: 33785119 Free PMC article. No abstract available.
References
-
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA wwwjamanetworkcom/journals/jama/fullarticle/2762997 (last accessed on 12 April 2020) 2020;323:1843–1844 . - PMC - PubMed
-
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 2000045. www.ncbi.nlm.nih.gov/pmc/articles/PMC6988269/ (last accessed on 12 April 2020) - PMC - PubMed
-
- Wikramaratna P, Paton RS, Ghafari M, Lourenco J. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. medRxiv. wwwdoiorg/101101/2020040520053355 (last accessed on 13 May 2020) 2020 - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

