Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis
- PMID: 32763266
- PMCID: PMC7749849
- DOI: 10.1016/j.jhep.2020.07.041
Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis
Abstract
Background & aims: Increased hepatocyte death contributes to the pathology of acute and chronic liver diseases. However, the role of hepatocyte pyroptosis and extracellular inflammasome release in liver disease is unknown.
Methods: We used primary mouse and human hepatocytes, hepatocyte-specific leucine 351 to proline Nlrp3KICreA mice, and GsdmdKO mice to investigate pyroptotic cell death in hepatocytes and its impact on liver inflammation and damage. Extracellular NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasomes were isolated from mutant NLRP3-YFP HEK cells and internalisation was studied in LX2 and primary human hepatic stellate cells. We also examined a cohort of 154 adult patients with biopsy-proven non-alcoholic fatty liver disease (Sir Charles Gairdner Hospital, Nedlands, Western Australia).
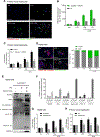
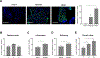
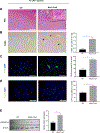
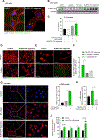
Results: We demonstrated that primary mouse and human hepatocytes can undergo pyroptosis upon NLRP3 inflammasome activation with subsequent release of NLRP3 inflammasome proteins that amplify and perpetuate inflammasome-driven fibrogenesis. Pyroptosis was inhibited by blocking caspase-1 and gasdermin D activation. The activated form of caspase-1 was detected in the livers and in serum from patients with non-alcoholic steatohepatitis and correlated with disease severity. Nlrp3KICreA mice showed spontaneous liver fibrosis under normal chow diet, and increased sensitivity to liver damage and inflammation after treatment with low dose lipopolysaccharide. Mechanistically, hepatic stellate cells engulfed extracellular NLRP3 inflammasome particles leading to increased IL-1β secretion and α-smooth muscle actin expression. This effect was abrogated when cells were pre-treated with the endocytosis inhibitor cytochalasin B.
Conclusions: These results identify hepatocyte pyroptosis and release of inflammasome components as a novel mechanism to propagate liver injury and liver fibrosis development.
Lay summary: Our findings identify a novel mechanism of inflammation in the liver. Experiments in cell cultures, mice, and human samples show that a specific form of cell death, called pyroptosis, leads to the release of complex inflammatory particles, the NLRP3 inflammasome, from inside hepatocytes into the extracellular space. From there they are taken up by other cells and thereby mediate inflammatory and pro-fibrogenic stress signals. The discovery of this mechanism may lead to novel treatments for chronic liver diseases in the future.
Keywords: ASC; Fibrosis; Hepatocytes; Inflammasome; Liver; NASH; NLRP3; Pyroptosis; Specks.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nature reviews. Immunology 2020;20(3):143–57. - PubMed
-
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012;481(7381):278–86. - PubMed
-
- Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nature reviews. Immunology 2016;16(7):407–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

