Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: A systematic review and meta-analysis
- PMID: 32763496
- PMCID: PMC7402237
- DOI: 10.1016/j.tmaid.2020.101825
Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: A systematic review and meta-analysis
Abstract
Introduction: Since December 2019, a novel coronavirus (SARS-CoV-2) has triggered a world-wide pandemic with an enormous medical and societal-economic toll. Thus, our aim was to gather all available information regarding comorbidities, clinical signs and symptoms, outcomes, laboratory findings, imaging features, and treatments in patients with coronavirus disease 2019 (COVID-19).
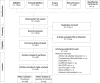
Methods: EMBASE, PubMed/Medline, Scopus, and Web of Science were searched for studies published in any language between December 1st, 2019 and March 28th, 2020. Original studies were included if the exposure of interest was an infection with SARS-CoV-2 or confirmed COVID-19. The primary outcome was the risk ratio of comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatments, outcomes, and complications associated with COVID-19 morbidity and mortality. We performed random-effects pairwise meta-analyses for proportions and relative risks, I2, T2, and Cochrane Q, sensitivity analyses, and assessed publication bias.
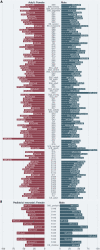
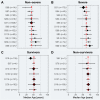
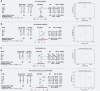
Results: 148 studies met the inclusion criteria for the systematic review and meta-analysis with 12'149 patients (5'739 female) and a median age of 47.0 [35.0-64.6] years. 617 patients died from COVID-19 and its complication. 297 patients were reported as asymptomatic. Older age (SMD: 1.25 [0.78-1.72]; p < 0.001), being male (RR = 1.32 [1.13-1.54], p = 0.005) and pre-existing comorbidity (RR = 1.69 [1.48-1.94]; p < 0.001) were identified as risk factors of in-hospital mortality. The heterogeneity between studies varied substantially (I2; range: 1.5-98.2%). Publication bias was only found in eight studies (Egger's test: p < 0.05).
Conclusions: Our meta-analyses revealed important risk factors that are associated with severity and mortality of COVID-19.
Keywords: COVID-19; Clinical characteristics; Comorbidities; Imaging features; Laboratory findings; Meta-analysis; Outcomes; SARS-CoV-2; Systematic review; Treatment.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors do not report any (financial or otherwise) conflict of interest.
Figures



Comment in
-
Histamine release theory and roles of antihistamine in the treatment of cytokines storm of COVID-19.Travel Med Infect Dis. 2020 Sep-Oct;37:101874. doi: 10.1016/j.tmaid.2020.101874. Epub 2020 Sep 3. Travel Med Infect Dis. 2020. PMID: 32891724 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

