Comparing a Mobile Phone Automated System With a Paper and Email Data Collection System: Substudy Within a Randomized Controlled Trial
- PMID: 32763873
- PMCID: PMC7479579
- DOI: 10.2196/15284
Comparing a Mobile Phone Automated System With a Paper and Email Data Collection System: Substudy Within a Randomized Controlled Trial
Abstract
Background: Traditional data collection methods using paper and email are increasingly being replaced by data collection using mobile phones, although there is limited evidence evaluating the impact of mobile phone technology as part of an automated research management system on data collection and health outcomes.
Objective: The aim of this study is to compare a web-based mobile phone automated system (MPAS) with a more traditional delivery and data collection system combining paper and email data collection (PEDC) in a cohort of breastfeeding women.
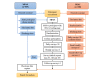
Methods: We conducted a substudy of a randomized controlled trial in Sydney, Australia, which included women with uncomplicated term births who intended to breastfeed. Women were recruited within 72 hours of giving birth. A quasi-randomized number of women were recruited using the PEDC system, and the remainder were recruited using the MPAS. The outcomes assessed included the effectiveness of data collection, impact on study outcomes, response rate, acceptability, and cost analysis between the MPAS and PEDC methods.
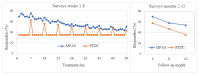
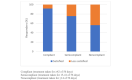
Results: Women were recruited between April 2015 and December 2016. The analysis included 555 women: 471 using the MPAS and 84 using the PEDC. There were no differences in clinical outcomes between the 2 groups. At the end of the 8-week treatment phase, the MPAS group showed an increased response rate compared with the PEDC group (56% vs 37%; P<.001), which was also seen at the 2-, 6-, and 12-month follow-ups. At the 2-month follow-up, the MPAS participants also showed an increased rate of self-reported treatment compliance (70% vs 56%; P<.001) and a higher recommendation rate for future use (95% vs 64%; P<.001) as compared with the PEDC group. The cost analysis between the 2 groups was comparable.
Conclusions: MPAS is an effective and acceptable method for improving the overall management, treatment compliance, and methodological quality of clinical research to ensure the validity and reliability of findings.
Keywords: breastfeeding; clinical trial; data collection methods; maternal health; mobile phones; text messaging.
©Diana M Bond, Jeremy Hammond, Antonia W Shand, Natasha Nassar. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 25.08.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Jüni P, Altman D, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. Br Med J. 2001 Jul 7;323(7303):42–6. doi: 10.1136/bmj.323.7303.42. http://europepmc.org/abstract/MED/11440947 - DOI - PMC - PubMed
-
- Sibbald B, Roland M. Understanding controlled trials. Why are randomised controlled trials important? Br Med J. 1998 Jan 17;316(7126):201. doi: 10.1136/bmj.316.7126.201. http://europepmc.org/abstract/MED/9468688 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

