Association of epileptiform abnormalities and seizures in Alzheimer disease
- PMID: 32764101
- PMCID: PMC7713786
- DOI: 10.1212/WNL.0000000000010612
Association of epileptiform abnormalities and seizures in Alzheimer disease
Abstract
Objective: To examine the relationship between scalp EEG biomarkers of hyperexcitability in Alzheimer disease (AD) and to determine how these electric biomarkers relate to the clinical expression of seizures in AD.
Methods: In this cross-sectional study, we performed 24-hour ambulatory scalp EEGs on 43 cognitively normal elderly healthy controls (HC), 41 participants with early-stage AD with no history or risk factors for epilepsy (AD-NoEp), and 15 participants with early-stage AD with late-onset epilepsy related to AD (AD-Ep). Two epileptologists blinded to diagnosis visually reviewed all EEGs and annotated all potential epileptiform abnormalities. A panel of 9 epileptologists blinded to diagnosis was then surveyed to generate a consensus interpretation of epileptiform abnormalities in each EEG.
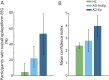
Results: Epileptiform abnormalities were seen in 53% of AD-Ep, 22% of AD-NoEp, and 4.7% of HC. Specific features of epileptiform discharges, including high frequency, robust morphology, right temporal location, and occurrence during wakefulness and REM, were associated with clinical seizures in AD. Multiple EEG biomarkers concordantly demonstrated a pattern of left temporal lobe hyperexcitability in early stages of AD, whereas clinical seizures in AD were often associated with bitemporal hyperexcitability. Frequent small sharp spikes were specifically associated with epileptiform EEGs and thus identified as a potential biomarker of hyperexcitability in AD.
Conclusion: Epileptiform abnormalities are common in AD but not all equivalent. Specific features of epileptiform discharges are associated with clinical seizures in AD. Given the difficulty recognizing clinical seizures in AD, these EEG features could provide guidance on which patients with AD are at high risk for clinical seizures.
© 2020 American Academy of Neurology.
Figures



Comment in
-
Restoring EEG to its Rightful Place in Alzheimer Disease Care.Epilepsy Curr. 2021 Mar 9;21(3):175-176. doi: 10.1177/1535759721998656. eCollection 2021 May-Jun. Epilepsy Curr. 2021. PMID: 34867098 Free PMC article. No abstract available.
References
-
- Busche MA, Konnerth A. Neuronal hyperactivity: a key defect in Alzheimer's disease? Bioessays 2015;37:624–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
