Accelerated Weathering Effects on Poly(3-hydroxybutyrate- co-3-hydroxyvalerate) (PHBV) and PHBV/TiO2 Nanocomposites
- PMID: 32764247
- PMCID: PMC7464598
- DOI: 10.3390/polym12081743
Accelerated Weathering Effects on Poly(3-hydroxybutyrate- co-3-hydroxyvalerate) (PHBV) and PHBV/TiO2 Nanocomposites
Abstract
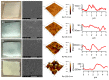
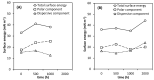
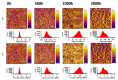
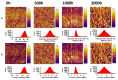
The effect of accelerated weathering on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and PHBV-based nanocomposites with rutile titanium (IV) dioxide (PHBV/TiO2) was investigated. The accelerated weathering test applied consecutive steps of UV irradiation (at 340 nm and 0.76 W m-2 irradiance) and moisture at 50 °C following the ASTM D4329 standard for up to 2000 h of exposure time. The morphology, chemical structure, crystallization, as well as the mechanical and thermal properties were studied. Samples were characterized after 500, 1000, and 2000 h of exposure time. Different degradation mechanisms were proposed to occur during the weathering exposure and were confirmed based on the experimental data. The PHBV surface revealed cracks and increasing roughness with the increasing exposure time, whereas the PHBV/TiO2 nanocomposites showed surface changes only after 2000 h of accelerated weathering. The degradation of neat PHBV under moisture and UV exposure occurred preferentially in the amorphous phase. In contrast, the presence of TiO2 in the nanocomposites retarded this process, but the degradation would occur simultaneously in both the amorphous and crystalline segments of the polymer after long exposure times. The thermal stability, as well as the temperature and rate of crystallization, decreased in the absence of TiO2. TiO2 not only provided UV protection, but also restricted the physical mobility of the polymer chains, acting as a nucleating agent during the crystallization process. It also slowed down the decrease in mechanical properties. The mechanical properties were shown to gradually decrease for the PHBV/TiO2 nanocomposites, whereas a sharp drop was observed for the neat PHBV after an accelerated weathering exposure. Atomic force microscopy (AFM), using the amplitude modulation-frequency modulation (AM-FM) tool, also confirmed the mechanical changes in the surface area of the PHBV and PHBV/TiO2 samples after accelerated weathering exposure. The changes in the physical and chemical properties of PHBV/TiO2 confirm the barrier activity of TiO2 for weathering attack and its retardation of the degradation process.
Keywords: accelerated weathering degradation; morphology and properties; poly(3-hydroxybutyrate-co-3-hydroxyvalerate); rutile titanium (IV) dioxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Tebaldi M.L., Maia A.L.C., Poletto F., De Andrade F.V., Soares D.C.F. Poly(-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Current advances in synthesis methodologies, antitumor applications and biocompatibility. J. Drug Deliv. Sci. Technol. 2019;51:115–126. doi: 10.1016/j.jddst.2019.02.007. - DOI
-
- Jiang L., Zhang J. Biodegradable Polymers and Polymer Blends. Elsevier; Amsterdam, The Netherlands: 2013. pp. 109–128.
-
- Li F., Yu H.-Y., Wang Y.-Y., Zhou Y., Zhang H., Yao J.-M., Abdalkarim S.Y.H., Tam K.C. Natural Biodegradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Nanocomposites with Multifunctional Cellulose Nanocrystals/Graphene Oxide Hybrids for High-Performance Food Packaging. J. Agric. Food Chem. 2019;67:10954–10967. doi: 10.1021/acs.jafc.9b03110. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

