Review of Drug Utilization Studies in Neonatal Units: A Global Perspective
- PMID: 32764503
- PMCID: PMC7459677
- DOI: 10.3390/ijerph17165669
Review of Drug Utilization Studies in Neonatal Units: A Global Perspective
Abstract
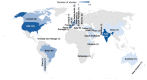
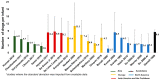
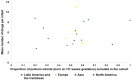
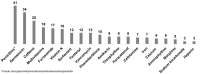
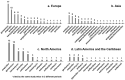
Rational prescribing is challenging in neonatology. Drug utilization studies help identify and define the problem. We performed a review of the literature on drug use in neonatal units and describe global variations. We searched databases (EMBASE, CINAHL and Medline) from inception to July 2020, screened studies and extracted relevant data (two reviewers). The search revealed 573 studies of which 84 were included. India (n = 14) and the USA (n = 13) reported the most. Data collection was prospective (n = 56) and retrospective (n = 26), mostly (n = 52) from one center only. Sixty studies described general drug use in 34 to 450,386 infants (median (IQR) 190 (91-767)) over a median (IQR) of 6 (3-18) months. Of the participants, 20-87% were preterm. The mean number of drugs per infant (range 11.1 to 1.7, pooled mean (SD) 4 (2.4)) was high with some reporting very high burden (≥30 drugs per infant in 8 studies). This was not associated with the proportion of preterm infants included. Antibiotics were the most frequently used drug. Drug use patterns were generally uniform with some variation in antibiotic use and more use of phenobarbitone in Asia. This study provides a global perspective on drug utilization in neonates and highlights the need for better quality information to assess rational prescribing.
Keywords: antibiotics; care; drug use review; infants; neonatal intensive; newborn.
Conflict of interest statement
Authors declare no conflict of interest. A.A.-T.—none to declare. I.C.—is on the Editorial Board of IJERPH. L.S.—none to declare. S.O.—has research funding from the National Institute of Health Research and The Medical Research Council, UK and local charities of the University Hospitals of Derby and Burton and the Nottingham University Hospitals NHS Trusts. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
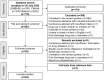
References
-
- World Health Organization . International Working Group for Drug Statistics Methodology, Organizzazione Mondiale Della Sanità, Collaborating Centre for Drug Statistics Methodology, Organizzazione Mondiale Della Sanità, Collaborating Centre for Drug Utilization Research and clinical Pharmacological Services. Introduction to Drug Utilization Research. World Health Organization; Geneva, Switzerland: 2003.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

