A FACS-based approach to obtain viable eosinophils from human adipose tissue
- PMID: 32764552
- PMCID: PMC7413382
- DOI: 10.1038/s41598-020-70093-z
A FACS-based approach to obtain viable eosinophils from human adipose tissue
Erratum in
-
Publisher Correction: A FACS-based approach to obtain viable eosinophils from human adipose tissue.Sci Rep. 2021 Sep 24;11(1):19434. doi: 10.1038/s41598-021-98825-9. Sci Rep. 2021. PMID: 34561525 Free PMC article. No abstract available.
Abstract
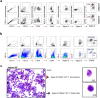
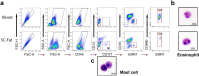
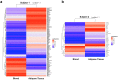
Eosinophils have been widely investigated in asthma and allergic diseases. More recently, new insights into the biology of these cells has illustrated eosinophils contribute to homeostatic functions in health such as regulation of adipose tissue glucose metabolism. Human translational studies are limited by the difficulty of obtaining cells taken directly from their tissue environment, relying instead on eosinophils isolated from peripheral blood. Isolation techniques for tissue-derived eosinophils can result in unwanted cell or ribonuclease activation, leading to poor cell viability or RNA quality, which may impair analysis of effector activities of these cells. Here we demonstrate a technique to obtain eosinophils from human adipose tissue samples for the purpose of downstream molecular analysis. From as little as 2 g of intact human adipose tissue, greater than 104 eosinophils were purified by fluorescence-activated cell sorting (FACS) protocol resulting in ≥ 99% purity and ≥ 95% viable eosinophils. We demonstrated that the isolated eosinophils could undergo epigenetic analysis to determine differences in DNA methylation in various settings. Here we focused on comparing eosinophils isolated from human peripheral blood vs human adipose tissue. Our results open the door to future mechanistic investigations to better understand the role of tissue resident eosinophils in different context.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

