Spontaneous membranoproliferative glomerulonephritis in a young Crl:CD-1(ICR) mouse
- PMID: 32764843
- PMCID: PMC7396734
- DOI: 10.1293/tox.2019-0081
Spontaneous membranoproliferative glomerulonephritis in a young Crl:CD-1(ICR) mouse
Abstract
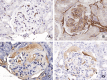
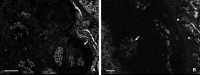
Here, we reported a spontaneous case of membranoproliferative glomerulonephritis observed in a young ICR mouse. A 5-week-old female mouse was euthanized owing to abdominal swelling and increased body weight. At necropsy, generalized subcutaneous edema, and clear, colorless, non-viscous ascites were observed. Histologically, the kidneys showed diffuse, bilateral glomerular lesions. The lesions were characterized by thickening and double contour of the basement membrane and an increase in mesangial cells and matrix, resulting in the narrowing of the capillary lumen. Additionally, eosinophilic hyaloid material accumulated in the subendothelial areas and Bowman's space. The material was positive for periodic acid-Schiff, complement component C3, or immunoglobulin G, stained red by Masson's trichrome, and stained blue by phosphotungstic acid-hematoxylin stain and was considered to be plasma due to glomerular leakage. The glomerular lesion was diagnosed as membranoproliferative glomerulonephritis, and an uncertain endothelial injury was suspected as the cause.
Keywords: CrlCD-1(ICR) mouse; membranoproliferative glomerulonephritis; spontaneous lesion.
©2020 The Japanese Society of Toxicologic Pathology.
Conflict of interest statement
Authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B, and Bube A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 40(4 Supple): 14S–86S. 2012. - PubMed
-
- Kouchi M, Toyosawa K, Matsumoto I, Michimae Y, Tochitani T, Koujitani T, Okimoto K, Funabashi H, and Seki T. Hyaline glomerulopathy with tubulo-fibrillary deposits in young ddY mice. Toxicol Pathol. 39: 975–979. 2011. - PubMed
-
- Raparia K, Usman I, and Kanwar YS. Renal morphologic lesions reminiscent of diabetic nephropathy. Arch Pathol Lab Med. 137: 351–359. 2013. - PubMed
-
- Matsui K, Sato H, Yokoyama Y, Egawa M, and Ito T. Nephrotic syndrome caused by thrombotic microangiopathy during bevacizumab treatment for lung metastases after rectal cancer surgery: A case report. Shimane J Med Sci. 35: 21–25. 2018.

