Nebulized Therapies in COPD: Past, Present, and the Future
- PMID: 32764912
- PMCID: PMC7367939
- DOI: 10.2147/COPD.S252435
Nebulized Therapies in COPD: Past, Present, and the Future
Abstract
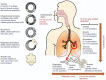
Current guidelines recommend inhalation therapy as the preferred route of drug administration for treating patients with chronic obstructive pulmonary disease (COPD). Inhalation devices consist of nebulizers and handheld inhalers, such as dry-powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs), and soft mist inhalers (SMIs). Although pMDIs, DPIs and SMIs may be appropriate for most patients with COPD, certain patient populations may have challenges with these devices. Patients who have cognitive, neuromuscular, or ventilatory impairments (and receive limited assistance from caregivers), as well as those with suboptimal peak inspiratory flow may not derive the full benefit from handheld inhalers. A considerable number of patients are not capable of producing a peak inspiratory flow rate to overcome the internal resistance of DPIs. Furthermore, patients may have difficulty coordinating inhalation with device actuation, which is required for pMDIs and SMIs. However, inhalation devices such as spacers and valved holding chambers can be used with pMDIs to increase the efficiency of aerosol delivery. Nebulized treatment provides patients with COPD an alternative administration route that avoids the need for inspiratory flow, manual dexterity, or complex hand-breath coordination. The recent approval of two nebulized long-acting muscarinic antagonists has added to the extensive range of nebulized therapies in COPD. Furthermore, with the availability of quieter and more portable nebulizer devices, nebulization may be a useful treatment option in the management of certain patient populations with COPD. The aim of this narrative review was to highlight recent updates and the treatment landscape in nebulized therapy and COPD. We first discuss the pathophysiology of patients with COPD and inhalation device considerations. Second, we review the updates on recently approved and newly marketed nebulized treatments, nebulized treatments currently in development, and technological advances in nebulizer devices. Finally, we discuss the current applications of nebulized therapy in patients with COPD.
Keywords: COPD; inhaler; nebulizer.
© 2020 Barjaktarevic and Milstone.
Conflict of interest statement
Dr Igor Z Barjaktarevic has no relevant conflicts related to the subject of this review. He has consulted with Astra Zeneca, Boehringer Ingelheim, CSL Behring, GE Healthcare, GlaxoSmithKline, Grifols, Mylan, Theravance Biopharma, and Verona Pharma. He has also received research grants from AMGEN, GE Healthcare, Mylan and Theravance Biopharma. Dr Aaron P Milstone has received speaking and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Grifols, Insmed, Mylan, and Theravance Biopharma.
Figures

References
-
- Centers for Disease Control and Prevention. COPD. Available from: https://www.cdc.gov/dotw/copd/index.html. Accessed May22, 2020.
-
- Centers for Disease Control and Prevention. National vital statistics reports. Final data for 2017. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_09-508.pdf. Accessed May12, 2020.
-
- COPD predicted to be third leading cause of death in 2030. World health stat; 2008. Available from: https://www.who.int/respiratory/copd/World_Health_Statistics_2008/en/. Accessed May12, 2020.
-
- American Lung Association. How serious is COPD; 2019. Available from: https://www.lung.org/lung-health-and-diseases/lung-disease-lookup/copd/l.... Accessed May12, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

