The Summit Score Stratifies Mortality and Morbidity in Chronic Obstructive Pulmonary Disease
- PMID: 32764918
- PMCID: PMC7381787
- DOI: 10.2147/COPD.S254437
The Summit Score Stratifies Mortality and Morbidity in Chronic Obstructive Pulmonary Disease
Abstract
Introduction: Tobacco use and other cardiovascular risk factors often accompany chronic obstructive pulmonary disease (COPD). This study derived and validated the Summit Score to predict mortality in people with COPD and cardiovascular risks.
Methods: SUMMIT trial subjects (N=16,485) ages 40-80 years with COPD were randomly assigned 50%/50% to derivation (N=8181) and internal validation (N=8304). Three external COPD validations from Intermountain Healthcare included outpatients with cardiovascular risks (N=9251), outpatients without cardiovascular risks (N=8551), and inpatients (N=26,170). Cox regression evaluated 40 predictors of all-cause mortality. SUMMIT treatments including combined fluticasone furoate (FF) 100μg/vilanterol 25μg (VI) were not included in the score.
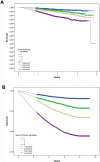
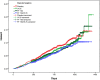
Results: Mortality predictors were FEV1, heart rate, systolic blood pressure, body mass index, age, smoking pack-years, prior COPD hospitalizations, myocardial infarction, heart failure, diabetes, anti-thrombotics, anti-arrhythmics, and xanthines. Combined in the Summit Score (derivation: c=0.668), quartile 4 vs 1 had HR=4.43 in SUMMIT validation (p<0.001, 95% CI=3.27, 6.01, c=0.662) and HR=8.15 in Intermountain cardiovascular risk COPD outpatients (p<0.001, 95% CI=5.86, 11.34, c=0.736), and strongly predicted mortality in the other Intermountain COPD populations. Among all SUMMIT subjects with scores 14-19, FF 100μg/VI 25μg vs placebo had HR=0.76 (p=0.0158, 95% CI=0.61, 0.95), but FF 100μg/VI 25μg was not different from placebo for scores <14 or >19.
Conclusion: In this post hoc analysis of SUMMIT trial data, the Summit Score was derived and validated in multiple Intermountain COPD populations. The score was used to identify a subpopulation in which mortality risk was lower for FF 100μg/VI 25μg treatment.
Trial registration: The SUMMIT trial is registered at ClinicalTrials.gov as number NCT01313676.
Keywords: IMRS; Intermountain risk score; clinical decision tool; randomized controlled trial; risk score.
© 2020 Horne et al.
Conflict of interest statement
BDH is an inventor of clinical decision tools that are licensed to CareCentra and Alluceo and is the PI of grants related to clinical decision tools that were funded by Intermountain Healthcare’s Foundry innovation program, the Intermountain Research and Medical Foundation, CareCentra, GlaxoSmithKline, and AstraZeneca. DPB and MH received research funding through AstraZeneca. DPB received grants outside the submitted work from ProLung and ZebraMedical. CC is employed by and holds restricted shares in GlaxoSmithKline. No other potential conflicts of interest exist.
Figures
References
-
- Vestbo J, Anderson JA, Brook RD, et al.; on behalf of the SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomized controlled trial. Lancet. 2016;387:1817–1826. doi:10.1016/S0140-6736(16)30069-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

