Molecular Basis for Pathogenicity of Human Coronaviruses
- PMID: 32765013
- PMCID: PMC7381773
- DOI: 10.2147/IDR.S255156
Molecular Basis for Pathogenicity of Human Coronaviruses
Abstract
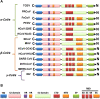
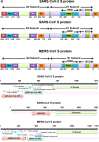
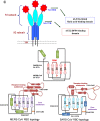
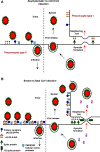
Over the past years, several zoonotic viruses have crossed the species barrier into humans and have been causing outbreaks of severe, and often fatal, respiratory illness. The 21st century has seen the worldwide spread of three recognized coronaviruses (CoVs) which can cause pneumonia and severe acute respiratory symptoms (SARSs), SARS, MERS, and recently SARS-CoV-2. Herein, it is raising concerns about the dissemination of another new and highly lethal pandemic outbreak. Preparing for a pandemic outbreak involves a great deal of awareness necessary to stop initial outbreaks, through recognizing the molecular mechanisms underlying virus transmission and pathogenicity. CoV spike protein S is the key determinant of host tropism and viral pathogenicity which can undergo variations and makes the CoV a highly pathogenic and diffusible virus capable of sustained human-to-human transmission and spread easily. The three mentioned CoVs exhibit some similarities in S protein whereby constitute a promising target for the development of prophylactics and therapeutics in the future.
Keywords: coronavirus; receptor binding; spike protein; transmission.
© 2020 Pourrajab et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- Ceccarelli M, Berretta M, Venanzi Rullo E, Nunnari G, Cacopardo B. Differences and similarities between severe acute respiratory syndrome (SARS)-coronavirus (CoV) and SARS-CoV-2. Would a rose by another name smell as sweet? Eur Rev Med Pharmacol Sci. 2020;24(5):2781–2783. doi: 10.26355/eurrev_202003_20551 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

