Efficacy, Practicality, and Safety of Inhaled Methoxyflurane in Elderly Patients with Acute Trauma Pain: Subgroup Analysis of a Randomized, Controlled, Multicenter, Open-Label Trial (MEDITA)
- PMID: 32765053
- PMCID: PMC7371437
- DOI: 10.2147/JPR.S255532
Efficacy, Practicality, and Safety of Inhaled Methoxyflurane in Elderly Patients with Acute Trauma Pain: Subgroup Analysis of a Randomized, Controlled, Multicenter, Open-Label Trial (MEDITA)
Abstract
Purpose: Acute trauma pain management in the elderly population is a challenge. Inhaled methoxyflurane represents a promising treatment option; however, data in the elderly population are limited.
Patients and methods: Subgroup, post hoc analysis including 69 patients aged ≥65 years from a randomized, active-controlled, open-label study in the emergency setting. Key inclusion criterion was moderate-to-severe pain (Numerical Rating Scale [NRS] score ≥ 4]) secondary to trauma in a single limb. Patients received inhaled methoxyflurane (3 mL) or standard analgesic treatment (SAT; IV paracetamol 1 g or ketoprofen 100 mg for moderate pain [NRS 4-6] and IV morphine 0.1mg/kg for severe pain [NRS ≥7]). The primary endpoint was the overall change in visual analog scale (VAS) pain intensity from randomization to the next 3, 5, and 10 min. Secondary endpoints included time to onset of pain relief (TOPR), efficacy up to 30 min, judgment of operators and patients, and safety.
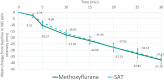
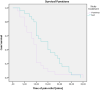
Results: Pain reduction over time was similar in both groups. Median TOPR was shorter for methoxyflurane (9 min; 95% CI: 7.8, 10.2 min) than SAT (15 min; 95% CI: 10.2, 19.8 min). In terms of treatment satisfaction, patients and operators rated treatment efficacy and practicality, respectively, as "Excellent" or "Very good" 5.7 times and 3.4 times more frequently than SAT. A similar rate of adverse events (methoxyflurane: 6 events; SAT: 7 events) was recorded, all non-serious. No clinically significant changes in vital signs parameters were observed, and methoxyflurane did not result in cases of bradycardia or hypotension.
Conclusion: In elderly patients with trauma pain, inhaled methoxyflurane shows similar pain relief and safety compared to SAT, offering advantages in terms of onset of effect and user's satisfaction. Although this analysis presents some methodological limitations, it provides the first specific evidence of the use of inhaled methoxyflurane in the elderly population.
Trial registration: EudraCT number: 2017-001565-25; Clinicaltrials.gov identifier NCT03585374.
Keywords: acute pain; analgesia; elderly; emergency department; methoxyflurane; prehospital; trauma.
© 2020 Serra et al.
Conflict of interest statement
Antonio Voza, Sossio Serra, Germana Ruggiano reports non-financial support from Mundipharma, Andrea Fabbri, have nothing to disclose. Elisabetta Bonafede is an employee of the clinical research organization that conducted the study. Antonella Sblendido, Amedeo Soldi and Alberto Farina are employees of Mundipharma Pharmaceuticals srl.
Figures

References
-
- Raccomandazioni Intersocietarie Italiane (SIAARTI, SIMEU, SIS 118, AISD, SIARED, SICUT, IRC) sulla gestione del dolore in emergenza. Available from: http://www.aisd.it/e107_files/downloads/raccintersocietarie_it_complete3.... Accessed February27, 2020.
-
- Hansen MS, Dahl JB. Limited evidence for intranasal fentanyl in the emergency department and the prehospital setting: a systematic review. Dan Med J. 2013;60:A4563. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

