Aberrant Cerebellar Circuitry in the Spinocerebellar Ataxias
- PMID: 32765211
- PMCID: PMC7378801
- DOI: 10.3389/fnins.2020.00707
Aberrant Cerebellar Circuitry in the Spinocerebellar Ataxias
Abstract
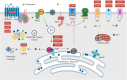
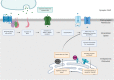
The spinocerebellar ataxias (SCAs) are a heterogeneous group of neurodegenerative diseases that share convergent disease features. A common symptom of these diseases is development of ataxia, involving impaired balance and motor coordination, usually stemming from cerebellar dysfunction and neurodegeneration. For most spinocerebellar ataxias, pathology can be attributed to an underlying gene mutation and the impaired function of the encoded protein through loss or gain-of-function effects. Strikingly, despite vast heterogeneity in the structure and function of disease-causing genes across the SCAs and the cellular processes affected, the downstream effects have considerable overlap, including alterations in cerebellar circuitry. Interestingly, aberrant function and degeneration of Purkinje cells, the major output neuronal population present within the cerebellum, precedes abnormalities in other neuronal populations within many SCAs, suggesting that Purkinje cells have increased vulnerability to cellular perturbations. Factors that are known to contribute to perturbed Purkinje cell function in spinocerebellar ataxias include altered gene expression resulting in altered expression or functionality of proteins and channels that modulate membrane potential, downstream impairments in intracellular calcium homeostasis and changes in glutamatergic input received from synapsing climbing or parallel fibers. This review will explore this enhanced vulnerability and the aberrant cerebellar circuitry linked with it in many forms of SCA. It is critical to understand why Purkinje cells are vulnerable to such insults and what overlapping pathogenic mechanisms are occurring across multiple SCAs, despite different underlying genetic mutations. Enhanced understanding of disease mechanisms will facilitate the development of treatments to prevent or slow progression of the underlying neurodegenerative processes, cerebellar atrophy and ataxic symptoms.
Keywords: Purkinje cell dysfunction; Purkinje cell vulnerability; cerebellar circuitry; cerebellar pathophysiology; disease mechanisms; neurodegeneration; spinocerebellar ataxia.
Copyright © 2020 Robinson, Watchon and Laird.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

