Quantitative Systems Pharmacology Model-Based Predictions of Clinical Endpoints to Optimize Warfarin and Rivaroxaban Anti-Thrombosis Therapy
- PMID: 32765265
- PMCID: PMC7381140
- DOI: 10.3389/fphar.2020.01041
Quantitative Systems Pharmacology Model-Based Predictions of Clinical Endpoints to Optimize Warfarin and Rivaroxaban Anti-Thrombosis Therapy
Abstract
Background: Tight monitoring of efficacy and safety of anticoagulants such as warfarin is imperative to optimize the benefit-risk ratio of anticoagulants in patients. The standard tests used are measurements of prothrombin time (PT), usually expressed as international normalized ratio (INR), and activated partial thromboplastin time (aPTT).
Objective: To leverage a previously developed quantitative systems pharmacology (QSP) model of the human coagulation network to predict INR and aPTT for warfarin and rivaroxaban, respectively.
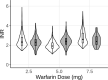
Methods: A modeling and simulation approach was used to predict INR and aPTT measurements of patients receiving steady-state anticoagulation therapy. A previously developed QSP model was leveraged for the present analysis. The effect of genetic polymorphisms known to influence dose response of warfarin (CYP2C9, VKORC1) were implemented into the model by modifying warfarin clearance (CYP2C9 *1: 0.2 L/h; *2: 0.14 L/h, *3: 0.04 L/h) and the concentration of available vitamin K (VKORC1 GA: -22% from normal vitamin K concentration; AA: -44% from normal vitamin K concentration). Virtual patient populations were used to assess the ability of the model to accurately predict routine INR and aPTT measurements from patients under long-term anticoagulant therapy.
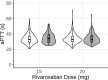
Results: The introduced model accurately described the observed INR of patients receiving long-term warfarin treatment. The model was able to demonstrate the influence of genetic polymorphisms of CYP2C9 and VKORC1 on the INR measurements. Additionally, the model was successfully used to predict aPTT measurements for patients receiving long-term rivaroxaban therapy.
Conclusion: The QSP model accurately predicted INR and aPTT measurements observed during routine therapeutic drug monitoring. This is an exemplar of how a QSP model can be adapted and used as a model-based precision dosing tool during clinical practice and drug development to predict efficacy and safety of anticoagulants to ultimately help optimize anti-thrombotic therapy.
Keywords: anticoagulation network; biomarker; precision dosing; quantitative systems pharmacology; rivaroxaban; warfarin.
Copyright © 2020 Hartmann, Biliouris, Lesko, Nowak-Göttl and Trame.
Figures

Similar articles
-
Leveraging QSP Models for MIPD: A Case Study for Warfarin/INR.Clin Pharmacol Ther. 2024 Sep;116(3):795-806. doi: 10.1002/cpt.3274. Epub 2024 Apr 24. Clin Pharmacol Ther. 2024. PMID: 38655898
-
Influence of CYP2C9, VKORC1, and CYP4F2 polymorphisms on the pharmacodynamic parameters of warfarin: a cross-sectional study.Pharmacol Rep. 2021 Oct;73(5):1405-1417. doi: 10.1007/s43440-021-00256-w. Epub 2021 Apr 3. Pharmacol Rep. 2021. PMID: 33811620
-
Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients.Eur J Clin Pharmacol. 2007 Dec;63(12):1135-41. doi: 10.1007/s00228-007-0381-6. Epub 2007 Sep 27. Eur J Clin Pharmacol. 2007. PMID: 17899045 Clinical Trial.
-
Bleeding with dabigatran, rivaroxaban, apixaban. No antidote, and little clinical experience.Prescrire Int. 2013 Jun;22(139):155-9. Prescrire Int. 2013. PMID: 23866358 Review.
-
Appraisal of current vitamin K dosing algorithms for the reversal of over-anticoagulation with warfarin: the need for a more tailored dosing regimen.Eur J Haematol. 2006 Dec;77(6):457-62. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2957.x. Epub 2006 Oct 17. Eur J Haematol. 2006. PMID: 17042764 Review.
Cited by
-
Asia-Inclusive Clinical Research and Development Enabled by Translational Science and Quantitative Clinical Pharmacology: Toward a Culture That Challenges the Status Quo.Clin Pharmacol Ther. 2023 Feb;113(2):298-309. doi: 10.1002/cpt.2591. Epub 2022 Apr 17. Clin Pharmacol Ther. 2023. PMID: 35342942 Free PMC article.
-
Deriving mechanism-based pharmacodynamic models by reducing quantitative systems pharmacology models: An application to warfarin.CPT Pharmacometrics Syst Pharmacol. 2023 Apr;12(4):432-443. doi: 10.1002/psp4.12903. Epub 2023 Mar 3. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36866520 Free PMC article.
-
The Combination of Cell Cultured Technology and In Silico Model to Inform the Drug Development.Pharmaceutics. 2021 May 12;13(5):704. doi: 10.3390/pharmaceutics13050704. Pharmaceutics. 2021. PMID: 34065907 Free PMC article. Review.
-
The effects of rivaroxaban, an oral anticoagulant, on human IVD primary cultures.Arch Med Sci. 2021 May 9;18(4):1062-1070. doi: 10.5114/aoms/136323. eCollection 2022. Arch Med Sci. 2021. PMID: 35832710 Free PMC article.
-
Simulating clinical trials for model-informed precision dosing: using warfarin treatment as a use case.Front Pharmacol. 2023 Oct 19;14:1270443. doi: 10.3389/fphar.2023.1270443. eCollection 2023. Front Pharmacol. 2023. PMID: 37927586 Free PMC article.
References
LinkOut - more resources
Full Text Sources

