Shexiang Baoxin Pill, a Traditional Chinese Herbal Formula, Rescues the Cognitive Impairments in APP/PS1 Transgenic Mice
- PMID: 32765267
- PMCID: PMC7381243
- DOI: 10.3389/fphar.2020.01045
Shexiang Baoxin Pill, a Traditional Chinese Herbal Formula, Rescues the Cognitive Impairments in APP/PS1 Transgenic Mice
Abstract
Background: Shexiang Baoxin Pill (SBP), a formulated traditional Chinese medicine (TCM), has been widely used to treat cardiovascular diseases for years. This herbal mixture has been shown to promote differentiation of cultured neuronal cells. Here, we aimed to investigate the effects of SBP in attenuating cognitive impairment in APP/PS1 transgenic mice.
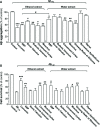
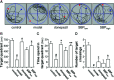
Methods: Ethanol and water extracts of SBP, denoted as SBPEtOH and SBPwater, were standardized and applied onto cultured rat pheochromocytoma PC12 cells. The potential effect of SBPEtOH extract in attenuating the cognitive impairments in APP/PS1 transgenic mice was shown by following lines of evidence: (i) inhibition of Aβ fibril formation, (ii) suppression of secretions of cytokines, and (iii) improvement of behavioral tests by Morris water maze.
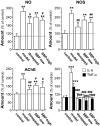
Results: SBPwater and SBPEtOH inhibited the formation of β-amyloid fibrils and protected the Aβ-induced cytotoxicity in cultured PC12 cells. In APP/PS1 transgenic mice, the treatment of SBPEtOH inhibited expressions of NO, NOS, AChE, as well as aggregation of Aβ. Besides, the levels of pro-inflammatory cytokines were suppressed by SBP treatment in the transgenic mice. Importantly, the behavioral tests by Morris Water maze indicated that SBP attenuated cognitive impairments in APP/PS1 transgenic mice.
Conclusion: The current result has supported the notion that SPB might ameliorate the cognitive impairment through multiple targets, suggesting that SBP could be considered as a promising anti-AD agent.
Keywords: Alzheimer’s disease; Chinese medicine; Shexiang Baoxin Pill; acetylcholinesterase; cognitive enhancing effect; neuroprotective.
Copyright © 2020 Hu, Mak, Zheng, Xia, Xu, Duan, Dong, Li, Zhan, Shang and Tsim.
Figures




Similar articles
-
Shexiang Baoxin Pill, a Formulated Chinese Herbal Mixture, Induces Neuronal Differentiation of PC12 Cells: A Signaling Triggered by Activation of Protein Kinase A.Front Pharmacol. 2019 Oct 9;10:1130. doi: 10.3389/fphar.2019.01130. eCollection 2019. Front Pharmacol. 2019. PMID: 31649530 Free PMC article.
-
Gardenia jasminoides J.Ellis extract GJ-4 alleviated cognitive deficits of APP/PS1 transgenic mice.Phytomedicine. 2021 Dec;93:153780. doi: 10.1016/j.phymed.2021.153780. Epub 2021 Sep 27. Phytomedicine. 2021. PMID: 34607163
-
Huatuo Zaizao pill ameliorates cognitive impairment of APP/PS1 transgenic mice by improving synaptic plasticity and reducing Aβ deposition.BMC Complement Altern Med. 2018 May 29;18(1):167. doi: 10.1186/s12906-018-2237-2. BMC Complement Altern Med. 2018. PMID: 29843688 Free PMC article.
-
Shenqi Xingnao Granules ameliorates cognitive impairments and Alzheimer's disease-like pathologies in APP/PS1 mouse model.Chin Herb Med. 2020 Oct 6;12(4):421-429. doi: 10.1016/j.chmed.2020.04.005. eCollection 2020 Oct. Chin Herb Med. 2020. PMID: 36120170 Free PMC article.
-
Shexiang Baoxin Pill, Derived From the Traditional Chinese Medicine, Provides Protective Roles Against Cardiovascular Diseases.Front Pharmacol. 2018 Nov 14;9:1161. doi: 10.3389/fphar.2018.01161. eCollection 2018. Front Pharmacol. 2018. PMID: 30487746 Free PMC article. Review.
Cited by
-
Shexiang Tongxin Dripping Pills regulates SOD/TNF-α/IL-6 pathway to inhibit inflammation and oxidative stress to improve myocardial ischemia-reperfusion injury in mice.Front Cardiovasc Med. 2025 Jun 12;12:1571925. doi: 10.3389/fcvm.2025.1571925. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40574818 Free PMC article.
-
Dracaena cochinchinensis stemwood extracts inhibit amyloid-β fibril formation and promote neuronal cell differentiation.Front Pharmacol. 2022 Sep 6;13:943638. doi: 10.3389/fphar.2022.943638. eCollection 2022. Front Pharmacol. 2022. PMID: 36147317 Free PMC article.
-
Effect of acupuncture on cognitive impairment induced by sleep deprivation in animal models: a preclinical systematic review and meta-analysis.Front Aging Neurosci. 2025 Mar 19;17:1560032. doi: 10.3389/fnagi.2025.1560032. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40177248 Free PMC article.
-
Ginsenosides attenuate bioenergetics and morphology of mitochondria in cultured PC12 cells under the insult of amyloid beta-peptide.J Ginseng Res. 2021 Jul;45(4):473-481. doi: 10.1016/j.jgr.2020.09.005. Epub 2020 Oct 1. J Ginseng Res. 2021. PMID: 34295207 Free PMC article.
-
Promising Strategies to Reduce the SARS-CoV-2 Amyloid Deposition in the Brain and Prevent COVID-19-Exacerbated Dementia and Alzheimer's Disease.Pharmaceuticals (Basel). 2024 Jun 16;17(6):788. doi: 10.3390/ph17060788. Pharmaceuticals (Basel). 2024. PMID: 38931455 Free PMC article. Review.
References
-
- Chen W., Zhong S., Wang J., Dong J., Wang X., Wang J. (2008). Effect of heart-protecting musk pill on expression of bcl-2, caspase-3 in cerebral ischemia reperfusion injury in rats. J. Emerg. Tradit. Chin. Med. 17, 1421–1446.
-
- Choi R. C. Y., Zhu J. T. T., Leung K. W., Chu G. K. Y., Xie H. Q. H., Chen V. P., et al. (2010). A flavonol glycoside, isolated from roots of Panax notoginseng, reduces amyloid-b-induced neurotoxicity in cultured neurons: signaling transduction and drug development for Alzheimer’s disease. J. Alzheimer Dis. 19, 795–811. 10.3233/JAD-2010-1293 - DOI - PubMed
LinkOut - more resources
Full Text Sources

