Effects of Sudden Drop in Salinity on Osmotic Pressure Regulation and Antioxidant Defense Mechanism of Scapharca subcrenata
- PMID: 32765306
- PMCID: PMC7379902
- DOI: 10.3389/fphys.2020.00884
Effects of Sudden Drop in Salinity on Osmotic Pressure Regulation and Antioxidant Defense Mechanism of Scapharca subcrenata
Abstract
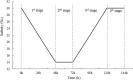
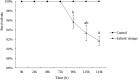
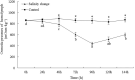
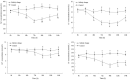
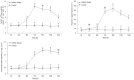
Salinity is an important ecological factor that impacts the growth and survival of aquatic organisms. The salinity of seawater in coastal and estuarine areas is often subject to dynamic changes because of seasonal rainfall and continental runoff. Thus, the current study investigated the effects of sudden changes in salinity on the survival rate and osmotic pressure regulation mechanisms of bottom-sowing seedlings of the economically important ark shell, Scapharca subcrenata. By simulating the sudden changes that occur in seawater salinity after rainstorms, the results showed that the osmotic pressure of the hemolymph and Na+, K+, Ca2+, and Cl- concentrations first decreased and then increased. When the salinity decreased from 30 to 14‰, hemoglobin, soluble total protein, taurine, and total free amino acid gradually increased; maximum levels of hemoglobin, soluble total protein, and taurine occurred once the salinity increased to 22‰ at 96 h. After 96 h, the total free amino acid content increased until 144 h. The reactive oxygen species (ROS) content and total antioxidant capacity (T-AOC) peaked at 96 h, whereas the expression levels of Mn-superoxide dismutase (MnSOD) and catalase (CAT) increased earlier, indicating that, with continuous ROS generation, antioxidant defense mechanisms were activated to avoid oxidative damage. Expression levels of cathepsin C (CTSC), cathepsin D (CTSD), heat shock protein 20 (HSP20), and heat shock protein 70 (HSP70) were significantly higher than in the control group at 48 h (salinity level 14‰); the expression levels of HSP20, heat shock protein 90 (HSP90), MnSOD, and glutathione peroxidase (GP x ) remained high, indicating that they were still required for osmotic pressure regulation to maintain the dynamic balance between the generation and removal of ROS as the salinity level increased. These results not only add to our basic understanding of the aquatic ecology of S. subcrenata, but also provide a theoretical ground for improving the survival rate of bottom-sowing, propagation, and release of S. subcrenata seedlings.
Keywords: Scapharca subcrenata; antioxidant defense; cathepsin; heat shock proteins; osmotic pressure; reactive oxygen species; salinity.
Copyright © 2020 Mo, Li, Ying and Xiaolong.
Figures


Similar articles
-
Effects of a Sudden Drop in Salinity on Scapharca subcrenata Antioxidant Defenses and Metabolism Determined Using LC-MS Non-targeted Metabolomics.Sci Rep. 2020 Apr 30;10(1):7324. doi: 10.1038/s41598-020-63293-0. Sci Rep. 2020. PMID: 32355228 Free PMC article.
-
Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters.Comp Biochem Physiol B Biochem Mol Biol. 2010 Jan;155(1):34-42. doi: 10.1016/j.cbpb.2009.09.008. Epub 2009 Sep 27. Comp Biochem Physiol B Biochem Mol Biol. 2010. PMID: 19788926
-
Transcriptomic analyses provide insights into the adaptive responses to heat stress in the ark shells, Scapharca subcrenata.Comp Biochem Physiol Part D Genomics Proteomics. 2021 Jun;38:100813. doi: 10.1016/j.cbd.2021.100813. Epub 2021 Feb 15. Comp Biochem Physiol Part D Genomics Proteomics. 2021. PMID: 33611220
-
Functional changes in hemocytes and antioxidant activity in gills of the ark clam Anadara kagoshimensis (Bivalvia: Arcidae) induced by salinity fluctuations.Comp Biochem Physiol B Biochem Mol Biol. 2023 Feb-Mar;264:110810. doi: 10.1016/j.cbpb.2022.110810. Epub 2022 Nov 4. Comp Biochem Physiol B Biochem Mol Biol. 2023. PMID: 36336300
-
Plant signaling networks involving Ca(2+) and Rboh/Nox-mediated ROS production under salinity stress.Front Plant Sci. 2015 Jun 10;6:427. doi: 10.3389/fpls.2015.00427. eCollection 2015. Front Plant Sci. 2015. PMID: 26113854 Free PMC article. Review.
Cited by
-
Transcriptomic Modulation Reveals the Specific Cellular Response in Chinese Sea Bass (Lateolabrax maculatus) Gills under Salinity Change and Alkalinity Stress.Int J Mol Sci. 2023 Mar 20;24(6):5877. doi: 10.3390/ijms24065877. Int J Mol Sci. 2023. PMID: 36982950 Free PMC article.
-
Multidimensional plasticity jointly contributes to rapid acclimation to environmental challenges during biological invasions.RNA. 2023 May;29(5):675-690. doi: 10.1261/rna.079319.122. Epub 2023 Feb 21. RNA. 2023. PMID: 36810233 Free PMC article.
-
Genome-Wide Identification and Expression Analysis of the ALDH Gene Family in Sinonovacula constricta Bivalve in Response to Acute Hypersaline Stress.Animals (Basel). 2024 Dec 30;15(1):64. doi: 10.3390/ani15010064. Animals (Basel). 2024. PMID: 39795007 Free PMC article.
-
Transcriptomic and Metabolomic Analyses Reveal Response Mechanisms of Sinonovacula Constricta to Saline-Alkalinity Stresses.Mar Biotechnol (NY). 2025 Mar 26;27(2):68. doi: 10.1007/s10126-025-10445-w. Mar Biotechnol (NY). 2025. PMID: 40138012
-
Genomic and Transcriptomic Landscape and Evolutionary Dynamics of Heat Shock Proteins in Spotted Sea Bass (Lateolabrax maculatus) under Salinity Change and Alkalinity Stress.Biology (Basel). 2022 Feb 23;11(3):353. doi: 10.3390/biology11030353. Biology (Basel). 2022. PMID: 35336727 Free PMC article.
References
-
- Abdel-Mohsen H. A. (2009). Assessment of respiratory and ion transport potential of Penaeus japonicus gills in response to environmental pollution. Mediterr. Mar. Sci. 10 5–18.
-
- Berger V. J., Kharazova A. D. (1997). Mechanisms of salinity adaptations in marine molluscs. Hydrobiologia 355 115–126. 10.1007/978-94-017-1907-0_12 - DOI
-
- Bhagat J., Ingole B. S., Singh N. (2016). Glutathione s-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as biomarkers of oxidative stress in snails: a review. ISJ 13 336–349.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

