A Zinc-Dependent Metalloproteinase of Brucella abortus Is Required in the Intracellular Adaptation of Macrophages
- PMID: 32765455
- PMCID: PMC7379133
- DOI: 10.3389/fmicb.2020.01586
A Zinc-Dependent Metalloproteinase of Brucella abortus Is Required in the Intracellular Adaptation of Macrophages
Abstract
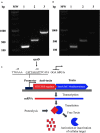
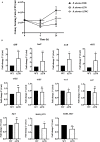
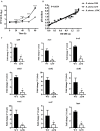
Brucella abortus is a pathogen that survives in macrophages. Several virulence factors participate in this process, including the open reading frame (ORF) BAB1_0270 codifying for a zinc-dependent metalloproteinase (ZnMP). Here, its contribution in the intracellular adaptation of B. abortus was analyzed by infecting RAW264.7 macrophages with the mutant B. abortus Δ270 strain. Results showed that this ZnMP did not participated in either the adherence or the initial intracellular traffic of B. abortus in macrophages. Nevertheless, its deletion significantly increased the co-localization of B. abortus Δ270 with phagolysosomal cathepsin D and reduced its co-localization with calnexin present in endoplasmic reticulum (RE)-derived vesicles. Although B. abortus Δ270 showed an upregulated expression of genes involved in virulence (vjbR, hutC, bvrR, virB1), it was insufficient to reach a successful intracellular replication within macrophages. Furthermore, its attenuation favored in macrophages infected the production of high levels of cytokines (TNF-α and IL-6) and co-stimulatory proteins (CD80 and CD86), signals required in T cell activation. Finally, its deletion significantly reduced the ability of B. abortus Δ270 to adapt, grow and express several virulence factors under acidic conditions. Based on these results, and considering that this ZnMP has homology with ImmA/IrrE proteases, we discuss its role in the virulence of this pathogen, concluding that ZnMP is required in the intracellular adaptation of B. abortus 2308 during the infection of macrophages.
Keywords: attenuation; gene regulation; host-pathogen interaction; toxin-anti-toxin systems; virulence.
Copyright © 2020 Gómez, Alvarez, Molina, Soto-Shara, Daza-Castro, Flores, León and Oñate.
Figures


References
-
- Arayan L. T., Simborio H. L., Reyes A. W., Hop H. T., Min W. G., Lee H. J., et al. (2015). The effects of red ginseng saponin fraction-A (RGSF-A) on phagocytosis and intracellular signaling in Brucella abortus infected RAW264.7 cells. FEMS Microbiol. Lett. 362:fnv070. 10.1093/femsle/fnv070 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

