The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses
- PMID: 32765522
- PMCID: PMC7378533
- DOI: 10.3389/fimmu.2020.01513
The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses
Abstract
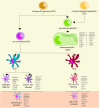
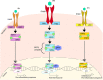
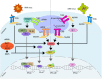
Dendritic cells (DCs) are a type of innate immune cells with major relevance in the establishment of an adaptive response, as they are responsible for the activation of lymphocytes. Since their discovery, several reports of their role during infectious diseases have been performed, highlighting their functions and their mechanisms of action. DCs can be categorized into different subsets, and each of these subsets expresses a wide arrange of receptors and molecules that aid them in the clearance of invading pathogens. Interferon (IFN) is a cytokine -a molecule of protein origin- strongly associated with antiviral immune responses. This cytokine is secreted by different cell types and is fundamental in the modulation of both innate and adaptive immune responses against viral infections. Particularly, DCs are one of the most important immune cells that produce IFN, with type I IFNs (α and β) highlighting as the most important, as they are associated with viral clearance. Type I IFN secretion can be induced via different pathways, activated by various components of the virus, such as surface proteins or genetic material. These molecules can trigger the activation of the IFN pathway trough surface receptors, including IFNAR, TLR4, or some intracellular receptors, such as TLR7, TLR9, and TLR3. Here, we discuss various types of dendritic cells found in humans and mice; their contribution to the activation of the antiviral response triggered by the secretion of IFN, through different routes of the induction for this important antiviral cytokine; and as to how DCs are involved in human infections that are considered highly frequent nowadays.
Keywords: IFN; antiviral response; dendritic cells; immune response; viruses.
Copyright © 2020 Soto, Gálvez, Andrade, Pacheco, Bohmwald, Berrios, Bueno and Kalergis.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

