Letrozole and the Traditional Chinese Medicine, Shaofu Zhuyu Decoction, Reduce Endometriotic Disease Progression in Rats: A Potential Role for Gut Microbiota
- PMID: 32765629
- PMCID: PMC7387974
- DOI: 10.1155/2020/3687498
Letrozole and the Traditional Chinese Medicine, Shaofu Zhuyu Decoction, Reduce Endometriotic Disease Progression in Rats: A Potential Role for Gut Microbiota
Abstract
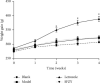
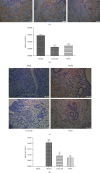
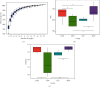
We previously showed that the Chinese herbal medicine, Shaofu Zhuyu decoction (SFZYD), shrank the size of endometriotic lesions in rats with endometriosis. We therefore conducted the present study to investigate the effects of letrozole and SFZYD on gut microbiota in endometriotic rats. Rats were divided into four groups: a blank group, model group, letrozole group, and SFZY group. Ectopic lesion size and COX-2 expression in the endometrium and endometriotic lesions were compared, and the community of gut microbiota was detected using 16S rRNA gene sequencing. Both letrozole and SFZYD reduced the size of ectopic lesions as well as lowered the expression of COX-2, thus reducing the inflammatory response. Compared with the blank group, the α-diversity of gut microbiota in endometriotic rats decreased, the Firmicutes/Bacteroidetes ratio increased, and the abundance of Ruminococcaceae was reduced. The α-diversity of gut microbiota in the letrozole group was similar to that in the model group, but the Firmicutes/Bacteroidetes ratio was diminished. The α-diversity in the SFZY group was similar to that in the blank group, the Firmicutes/Bacteroidetes ratio was attenuated, and the abundance of Ruminococcaceae was elevated compared with the model group. These results indicated that the therapeutic mechanisms of both letrozole and SFZYD were related to the restoration of gut microbiota.
Copyright © 2020 Ying Cao et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest in this study.
Figures


References
-
- Sampson J. A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. American Journal of Obstetrics and Gynecology. 1927;14(4):422–469. doi: 10.1016/s0002-9378(15)30003-x. - DOI
-
- Ahn S. H., Edwards A. K., Singh S. S., Young S. L., Lessey B. A., Tayade C. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. The Journal of Immunology. 2015;195(6):2591–2600. doi: 10.4049/jimmunol.1501138. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

