Drug Therapies for the Management of Sickle Cell Disease
- PMID: 32765834
- PMCID: PMC7388199
- DOI: 10.12688/f1000research.22433.1
Drug Therapies for the Management of Sickle Cell Disease
Abstract
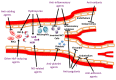
Sickle cell disease (SCD) afflicts millions of people worldwide but is referred to as an orphan disease in the United States. Over the past several decades, there has been an increasing understanding of the pathophysiology of SCD and its complications. While most individuals with SCD in resource-rich countries survive into adulthood, the life expectancy of patients with SCD remains substantially shorter than for the general African-American population. SCD can be cured using hematopoietic stem cell transplantation and possibly gene therapy, but these treatment approaches are not available to most patients, the majority of whom reside in low- and middle-income countries. Until relatively recently, only one drug, hydroxyurea, was approved by the US Food and Drug Administration to ameliorate disease severity. Multiple other drugs (L-glutamine, crizanlizumab, and voxelotor) have recently been approved for the treatment of SCD, with several others at various stages of clinical testing. The availability of multiple agents to treat SCD raises questions related to the choice of appropriate drug therapy, combination of multiple agents, and affordability of recently approved products. The enthusiasm for new drug development provides opportunities to involve patients in low- and middle-income nations in the testing of potentially disease-modifying therapies and has the potential to contribute to capacity building in these environments. Demonstration that these agents, alone or in combination, can prevent or decrease end-organ damage would provide additional evidence for the role of drug therapies in improving outcomes in SCD.
Keywords: Clinical Trials; Drug Development; Novel Drugs; Sickle cell disease; Treatment.
Copyright: © 2020 Rai P and Ataga KI.
Conflict of interest statement
Competing interests: KIA has served on clinical advisory boards and/or served as a consultant for Global Blood Therapeutics, Novartis, Emmaus Life Sciences, Editas Medicine, and Novo Nordisk. PR has no competing interests.Competing interests: YS has patents, owns equity in, and serves on the board of a company EpiDestiny that is developing drug therapy for sickle cell disease. No competing interests were disclosed.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous