Impact of a Maternal Prevention of Mother-to-child Transmission of HIV (PMTCT) Intervention on HIV-exposed Infants in Uganda
- PMID: 32765963
- PMCID: PMC7397331
- DOI: 10.21106/ijma.380
Impact of a Maternal Prevention of Mother-to-child Transmission of HIV (PMTCT) Intervention on HIV-exposed Infants in Uganda
Abstract
Background: Uganda has successfully reduced pediatric HIV infections through prevention of mother-to-child transmission of HIV (PMTCT) programs, yet little is known about adherence to infant-specific components of interventions. We hypothesized that infants born to mothers receiving the WiseMama (WM) electronic drug monitoring (EDM)-based adherence intervention would have increased uptake of six-week post-natal nevirapine (NVP) infant prophylaxis and better adherence to six-week early infant diagnosis (EID) HIV testing.
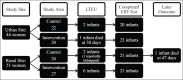
Methods: At two sites in Uganda, the Wise Infant Study (WIN) prospectively followed an infant cohort. Infants were born to women enrolled in an RCT testing the effect of real-time reminders delivered via EDM on maternal adherence to antiretroviral therapy. We assessed intrapartum and discharge receipt of NVP prophylaxis using pharmacy and infant HIV DNA testing laboratory data.
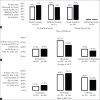
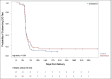
Results: Of 121 women eligible for WIN, 97 (80%) consented and enrolled; 46 had been randomized to control and 51 to intervention. There were no differences in receipt of a six-week NVP supply (control 87%, intervention 82%, p = 0.53). Receipt of any NVP prophylaxis did not vary by delivery location (p = 0.35), and although 12% of infants were delivered at non-study health facilities, they were not less likely to receive NVP at discharge (p = 0.37). Among infants with a completed HIV test, there was no difference in mean time to first test (control 52 days (SD 18), intervention 51 days (SD 15), p = 0.86). Only one infant, in the control group, tested positive for HIV.
Conclusion and global health implications: We found no significant differences in adherence to infant PMTCT practices between intervention and control infants with relatively high rates of NVP receipt albeit with suboptimal adherence to six-week EID testing. Further work is needed to ensure improved access, uptake, and follow-up of HIV-exposed infants in the Option B+ era.
Keywords: Antiretroviral therapy prophylaxis; Early infant diagnosis; HIV; HIV-exposed infants; Nevirapine; Prevention of maternal to child transmission of HIV.
Copyright © 2020 Murillo et al.
Conflict of interest statement
Conflicts of Interest: No conflicts of interest for any authors to disclose.
Figures
References
-
- United Nations Children's Fund. For Every Child, End AIDS:Seventh Stocktaking Report, 2016. 2016. Dec, [Accessed April 3 2020]. https://www.unicef.org/publications/
-
- Fowler MG, Lampe MA, Jamieson DJ, et al. Reducing the risk of mother-to-child human immunodeficiency virus transmission:past successes, current progress and challenges, and future directions. American Jounral of Obstetrics and Gynecology. 2007;197(3 Suppl):S3–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
