Dynamically Shaping Chaperones. Allosteric Modulators of HSP90 Family as Regulatory Tools of Cell Metabolism in Neoplastic Progression
- PMID: 32766157
- PMCID: PMC7378685
- DOI: 10.3389/fonc.2020.01177
Dynamically Shaping Chaperones. Allosteric Modulators of HSP90 Family as Regulatory Tools of Cell Metabolism in Neoplastic Progression
Abstract
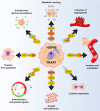
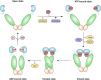
Molecular chaperones have recently emerged as fundamental regulators of salient biological routines, including metabolic adaptations to environmental changes. Yet, many of the molecular mechanisms at the basis of their functions are still unknown or at least uncertain. This is in part due to the lack of chemical tools that can interact with the chaperones to induce measurable functional perturbations. In this context, the use of small molecules as modulators of protein functions has proven relevant for the investigation of a number of biomolecular systems. Herein, we focus on the functions, interactions and signaling pathways of the HSP90 family of molecular chaperones as possible targets for the discovery of new molecular entities aimed at tuning their activity and interactions. HSP90 and its mitochondrial paralog, TRAP1, regulate the activity of crucial metabolic circuitries, making cells capable of efficiently using available energy sources, with relevant implications both in healthy conditions and in a variety of disease states and especially cancer. The design of small-molecules targeting the chaperone cycle of HSP90 and able to inhibit or stimulate the activity of the protein can provide opportunities to finely dissect their biochemical activities and to obtain lead compounds to develop novel, mechanism-based drugs.
Keywords: ATP-competitive inhibitors; HSP90; TRAP1; allosteric inhibitor; anti-neoplastic strategies; chaperones; mitochondria; tumor metabolism.
Copyright © 2020 Sanchez-Martin, Serapian, Colombo and Rasola.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

