Optimizing G6PD testing for Plasmodium vivax case management and beyond: why sex, counseling, and community engagement matter
- PMID: 32766454
- PMCID: PMC7388194
- DOI: 10.12688/wellcomeopenres.15700.2
Optimizing G6PD testing for Plasmodium vivax case management and beyond: why sex, counseling, and community engagement matter
Abstract
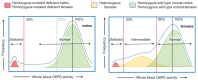
Safe access to the most effective treatment options for Plasmodium vivax malaria are limited by the absence of accurate point-of-care testing to detect glucose-6-phosphate dehydrogenase (G6PD) deficiency, the most common human genetic disorder. G6PD-deficient patients are at risk of life-threatening hemolysis when exposed to 8-aminoquinolines, the only class of drugs efficacious against P. vivax hypnozoites. Until recently, only qualitative tests were available in most settings. These can identify patients with severe G6PD deficiency (mostly male) but not patients with intermediate G6PD deficiency (always female). This has led to and reinforced a gap in awareness in clinical practice of the risks and implications of G6PD deficiency in females-who, unlike males, can have a heterozygous genotype for G6PD. Increasing recognition of the need for radical cure of P. vivax, first for patients' health and then for malaria elimination, is driving the development of new point-of-care tests for G6PD deficiency and their accessibility to populations in low-resource settings. The availability of user-friendly, affordable, and accurate quantitative point-of-care diagnostics for the precise classification of the three G6PD phenotypes can reduce sex-linked disparities by ensuring safe and effective malaria treatment, providing opportunities to develop supportive counseling to enhance understanding of genetic test results, and improving the detection of all G6PD deficiency phenotypes in newborns and their family members.
Keywords: G6PD deficiency; G6PD heterozygous females; G6PD testing; Plasmodium vivax; disparity; gender; genetic counselling; haemolysis; neonatal hyperbilirubinaemia; primaquine; sex; tafenoquine.
Copyright: © 2020 Chu CS et al.
Conflict of interest statement
Competing interests: PATH supports a portfolio of G6PD diagnostic test development efforts. PATH has no financial interests in the commercialisation of any resulting products.
Figures
References
-
- (WHO), W.H.O: World Malaria Report 2018.2018. Reference Source
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

