Early-stage sugar beet taproot development is characterized by three distinct physiological phases
- PMID: 32766510
- PMCID: PMC7395582
- DOI: 10.1002/pld3.221
Early-stage sugar beet taproot development is characterized by three distinct physiological phases
Abstract
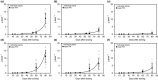
Despite the agronomic importance of sugar beet (Beta vulgaris L.), the early-stage development of its taproot has only been poorly investigated. Thus, the mechanisms that determine growth and sugar accumulation in sugar beet are largely unknown. In the presented study, a physiological characterization of early-stage sugar beet taproot development was conducted. Activities were analyzed for fourteen key enzymes of carbohydrate metabolism in developing taproots over the first 80 days after sowing. In addition, we performed in situ localizations of selected carbohydrate-metabolic enzyme activities, anatomical investigations, and quantifications of soluble carbohydrates, hexose phosphates, and phytohormones. Based on the accumulation dynamics of biomass and sucrose, as well as on anatomical parameters, the early phase of taproot development could be subdivided into three stages-prestorage, transition, secondary growth and sucrose accumulation stage-each of which was characterized by distinct metabolic and phytohormonal signatures. The enzyme activity signatures corresponding to these stages were also shown to be robustly reproducible in experiments conducted in two additional locations. The results from this physiological phenotyping approach contribute to the identification of the key regulators of sugar beet taproot development and open up new perspectives for sugar beet crop improvement concerning both physiological marker-based breeding and biotechnological approaches.
Keywords: assimilate partitioning; carbohydrate metabolism; developmental regulation; physiological phenotyping; sucrose accumulation; taproot development.
© 2020 The Authors. Plant Direct published by American Society of Plant Biologists, Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Appeldoorn, N. J. G. , de Bruijn, S. M. , Koot‐Gronsveld, E. A. M. , Visser, R. G. F. , Vreugdenhil, D. , & van der Plas, L. H. W. (1997). Developmental changes of enzymes involved in conversion of sucrose to hexose‐phosphate during early tuberisation of potato. Planta, 202, 220–226. 10.1007/s004250050122 - DOI
-
- Artschwager, E. (1926). Anatomy of the vegetative organs of the sugar beet. Journal of Agricultural Research, 33, 143–176.
-
- Artschwager, E. (1930). A study of the structure of sugar beets in relation to sugar content and type. Journal of Agricultural Research, 40, 867–915.
-
- Beauvoit, B. P. , Colombié, S. , Monier, A. , Andrieu, M.‐H. , Biais, B. , Bénard, C. , … Gibon, Y. (2014). Model‐assisted analysis of sugar metabolism throughout tomato fruit development reveals enzyme and carrier properties in relation to vacuole expansion. The Plant Cell, 26, 3224–3242. 10.1105/tpc.114.127761 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

