SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence
- PMID: 32766833
- PMCID: PMC7454740
- DOI: 10.1093/infdis/jiaa479
SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence
Abstract
Background: The COVID-19 pandemic necessitates better understanding of the kinetics of antibody production induced by infection with SARS-CoV-2. We aimed to develop a high-throughput multiplex assay to detect antibodies to SARS-CoV-2 to assess immunity to the virus in the general population.
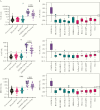
Methods: Spike protein subunits S1 and receptor binding domain, and nucleoprotein were coupled to microspheres. Sera collected before emergence of SARS-CoV-2 (n = 224) and of non-SARS-CoV-2 influenza-like illness (n = 184), and laboratory-confirmed cases of SARS-CoV-2 infection (n = 115) with various severities of COVID-19 were tested for SARS-CoV-2-specific IgG concentrations.
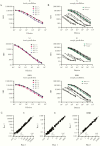
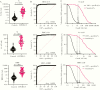
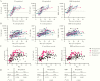
Results: Our assay discriminated SARS-CoV-2-induced antibodies and those induced by other viruses. The assay specificity was 95.1%-99.0% with sensitivity 83.6%-95.7%. By merging the test results for all 3 antigens a specificity of 100% was achieved with a sensitivity of at least 90%. Hospitalized COVID-19 patients developed higher IgG concentrations and the rate of IgG production increased faster compared to nonhospitalized cases.
Conclusions: The bead-based serological assay for quantitation of SARS-CoV-2-specific antibodies proved to be robust and can be conducted in many laboratories. We demonstrated that testing of antibodies against multiple antigens increases sensitivity and specificity compared to single-antigen-specific IgG determination.
Keywords: COVID-19; IgG; RBD; endemic coronavirus; influenza-like Illness (ILI); multiplex bead-based immune assay; nucleoprotein; sensitivity; specificity; spike S1.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- den Hartog G, van Binnendijk R, Buisman AM, Berbers GAM, van der Klis FRM. Immune surveillance for vaccine-preventable diseases. Expert Rev Vaccines 2020; 19:327–39. - PubMed
-
- GeurtsvanKessel CH, Okba NMA, Igloi Z, et al. . Towards the next phase: evaluation of serological assays for diagnostics and exposure assessment. medRxiv 20077156 [Preprint]. 5. May 2020. [cited 31 July2020]. Available from: 10.1101/2020.04.23.20077156. - DOI
-
- Lan J, Ge J, Yu J, et al. . Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020; 581:215–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

