Mechanistic insights and potential therapeutic approaches for NUP98-rearranged hematologic malignancies
- PMID: 32766874
- PMCID: PMC7702474
- DOI: 10.1182/blood.2020007093
Mechanistic insights and potential therapeutic approaches for NUP98-rearranged hematologic malignancies
Abstract
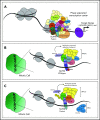
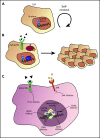
Nucleoporin 98 (NUP98) fusion oncoproteins are observed in a spectrum of hematologic malignancies, particularly pediatric leukemias with poor patient outcomes. Although wild-type full-length NUP98 is a member of the nuclear pore complex, the chromosomal translocations leading to NUP98 gene fusions involve the intrinsically disordered and N-terminal region of NUP98 with over 30 partner genes. Fusion partners include several genes bearing homeodomains or having known roles in transcriptional or epigenetic regulation. Based on data in both experimental models and patient samples, NUP98 fusion oncoprotein-driven leukemogenesis is mediated by changes in chromatin structure and gene expression. Multiple cofactors associate with NUP98 fusion oncoproteins to mediate transcriptional changes possibly via phase separation, in a manner likely dependent on the fusion partner. NUP98 gene fusions co-occur with a set of additional mutations, including FLT3-internal tandem duplication and other events contributing to increased proliferation. To improve the currently dire outcomes for patients with NUP98-rearranged malignancies, therapeutic strategies have been considered that target transcriptional and epigenetic machinery, cooperating alterations, and signaling or cell-cycle pathways. With the development of more faithful experimental systems and continued study, we anticipate great strides in our understanding of the molecular mechanisms and therapeutic vulnerabilities at play in NUP98-rearranged models. Taken together, these studies should lead to improved clinical outcomes for NUP98-rearranged leukemia.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.G.M. has received research fuding from Loxo Oncology, Pfizer and Illumina; speaking fees from Amgen, served on an advisory board for Illumina, and holds stock in Amgen. The remaining authors declare no competing financial interests.
Figures


References
-
- Harrison CJ, Hills RK, Moorman AV, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol. 2010;28(16):2674-2681. - PubMed
-
- Bolouri H, Farrar JE, Triche T Jr., et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions [published corrections appear in Nat Med. 2018;24(4):526 and Nat Med. 2019;25(3):530]. Nat Med. 2018;24(1):103-112. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

