Postdischarge thrombosis and hemorrhage in patients with COVID-19
- PMID: 32766883
- PMCID: PMC7483433
- DOI: 10.1182/blood.2020007938
Postdischarge thrombosis and hemorrhage in patients with COVID-19
Abstract
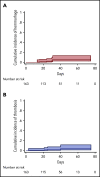
Coronavirus disease 2019 (COVID-19) is associated with a prothrombotic state with a high incidence of thrombotic events during hospitalization; however, data examining rates of thrombosis after discharge are limited. We conducted a retrospective observational cohort study of discharged patients with confirmed COVID-19 not receiving anticoagulation. The cohort included 163 patients with median time from discharge to last recorded follow-up of 30 days (interquartile range [IQR], 17-46 days). The median duration of index hospitalization was 6 days (IQR, 3-12 days) and 26% required intensive care. The cumulative incidence of thrombosis (including arterial and venous events) at day 30 following discharge was 2.5% (95% confidence interval [CI], 0.8-7.6); the cumulative incidence of venous thromboembolism alone at day 30 postdischarge was 0.6% (95% CI, 0.1-4.6). The 30-day cumulative incidence of major hemorrhage was 0.7% (95% CI, 0.1-5.1) and of clinically relevant nonmajor bleeds was 2.9% (95% CI, 1.0-9.1). We conclude that the rates of thrombosis and hemorrhage appear to be similar following hospital discharge for COVID-19, emphasizing the need for randomized data to inform recommendations for universal postdischarge thromboprophylaxis.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.I.Z. reports research funding from Incyte and Quercegen; consultancy services to Sanofi, CSL, and Parexel; and honoraria from/advisory board participation with Pfizer/Bristol Myers Squibb (BMS), Portola, and Daiichi. K.A.B. reports consultancy with BMS and Takeda. W.C.A. is employed by DynaMed. The remaining authors declare no competing financial interests.
Figures
References
-
- Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950-2973. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical