Synergistic Effect of Laminin and Epidermal Growth Factor on Biological and Morphological Properties of Co-Cultured Myoblasts and Fibroblasts
- PMID: 32767029
- PMCID: PMC7710774
- DOI: 10.1007/s13770-020-00283-3
Synergistic Effect of Laminin and Epidermal Growth Factor on Biological and Morphological Properties of Co-Cultured Myoblasts and Fibroblasts
Abstract
Background: One of the long-standing problems of myoblasts in vitro expansion is slow cell migration and this causes fibroblast population to exceed myoblasts. In this study, we investigated the synergistic effect of laminin and epidermal growth factor (EGF) on co-cultured myoblasts and fibroblasts for cell attachment, proliferation and migration.
Methods: Skeletal human muscle cells were cultured in four different conditions; control, EGF, laminin (Lam) and laminin EGF (Lam + EGF). Using live imaging system, their cellular properties; attachment, migration and growth were exposed to Rho kinase inhibitor, Y-27632, and EGF-receptor (EGF-R) inhibitor, gefitinib were measured.
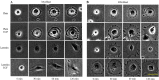
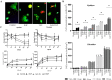
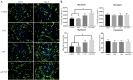
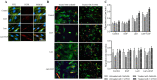
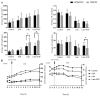
Results: Myoblast migration and proliferation was enhanced significantly by synergistic stimulation of laminin and EGF (0.61 ± 0.14 µm/min, 0.008 ± 0.001 h-1) compare to that by EGF alone (0.26 ± 0.13 µm/min, 0.004 ± 0.0009 h-1). However, no changes in proliferation and migration were observed for fibroblasts among the culture conditions. Inhibition of Rho kinase resulted in the increase of the myoblast migration on the laminin-coated surface with EGF condition (0.64 ± 0.18 µm/min). Compared to the untreated conditions, myoblasts cultured on the laminin-coated surface and EGF demonstrated elongated morphology, and average cell length increase significantly. In contrast, inhibition of EGF-R resulted in the decrease of myoblast migration on the laminin coated surface with EGF supplemented condition (0.43 ± 0.05 µm/min) in comparison to the untreated control (0.53 ± 0.05 µm/min).
Conclusion: Laminin and EGF preferentially enhance the proliferation and migration of myoblasts, and Rho kinase and EGF-R play a role in this synergistic effect. These results will be beneficial for the propagation of skeletal muscle cells for clinical applications.
Keywords: Epidermal growth factor; Gefitinib; Laminin; Myoblast.
Conflict of interest statement
The authors of this manuscript declare that they have no competing interests.
Figures
Similar articles
-
Laminin-Coated Poly(Methyl Methacrylate) (PMMA) Nanofiber Scaffold Facilitates the Enrichment of Skeletal Muscle Myoblast Population.Int J Mol Sci. 2017 Oct 30;18(11):2242. doi: 10.3390/ijms18112242. Int J Mol Sci. 2017. PMID: 29084180 Free PMC article.
-
Synergic stimulation of laminin and epidermal growth factor facilitates the myoblast growth through promoting migration.J Biosci Bioeng. 2009 Aug;108(2):174-7. doi: 10.1016/j.jbiosc.2009.03.005. J Biosci Bioeng. 2009. PMID: 19619867
-
One-Step Purification of Human Skeletal Muscle Myoblasts and Subsequent Expansion Using Laminin-Coated Surface.Tissue Eng Part C Methods. 2015 Nov;21(11):1135-42. doi: 10.1089/ten.TEC.2015.0015. Epub 2015 Jul 23. Tissue Eng Part C Methods. 2015. PMID: 26061720
-
Laminin responsiveness is associated with changes in fibroblast morphology, motility, and anchorage-independent growth: cell system for examining the interaction between laminin and EGF signaling pathways.J Cell Physiol. 1995 Sep;164(3):593-604. doi: 10.1002/jcp.1041640318. J Cell Physiol. 1995. PMID: 7650067
-
HGF potentiates extracellular matrix-driven migration of human myoblasts: involvement of matrix metalloproteinases and MAPK/ERK pathway.Skelet Muscle. 2017 Oct 10;7(1):20. doi: 10.1186/s13395-017-0138-6. Skelet Muscle. 2017. PMID: 29017538 Free PMC article.
Cited by
-
Electrospun Fiber-Coated Human Amniotic Membrane: A Potential Angioinductive Scaffold for Ischemic Tissue Repair.Int J Mol Sci. 2022 Feb 3;23(3):1743. doi: 10.3390/ijms23031743. Int J Mol Sci. 2022. PMID: 35163664 Free PMC article.
-
Optimization of skeletal muscle-derived fibroblast isolation and purification without the preplating method.Cell Tissue Bank. 2022 Sep;23(3):557-568. doi: 10.1007/s10561-021-09989-7. Epub 2022 Jan 25. Cell Tissue Bank. 2022. PMID: 35076859
References
-
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–7. - PubMed
-
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. - PubMed
-
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. - PubMed
-
- McGarvey ML, Baron-Van Evercooren A, Kleinman HK, Dubois-Dalcq M. Synthesis and effects of basement membrane components in cultured rat Schwann cells. Dev Biol. 1984;105:18–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
