Clinical outcomes after anterior cruciate ligament injury: panther symposium ACL injury clinical outcomes consensus group
- PMID: 32767052
- PMCID: PMC7429530
- DOI: 10.1007/s00167-020-06061-x
Clinical outcomes after anterior cruciate ligament injury: panther symposium ACL injury clinical outcomes consensus group
Abstract
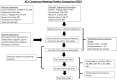
Purpose: A stringent outcome assessment is a key aspect for establishing evidence-based clinical guidelines for anterior cruciate ligament (ACL) injury treatment. The aim of this consensus statement was to establish what data should be reported when conducting an ACL outcome study, what specific outcome measurements should be used and at what follow-up time those outcomes should be assessed.
Methods: To establish a standardized approach to assessment of clinical outcome after ACL treatment, a consensus meeting including a multidisciplinary group of ACL experts was held at the ACL Consensus Meeting Panther Symposium, Pittsburgh, PA; USA, in June 2019. The group reached consensus on nine statements by using a modified Delphi method.
Results: In general, outcomes after ACL treatment can be divided into four robust categories-early adverse events, patient-reported outcomes, ACL graft failure/recurrent ligament disruption and clinical measures of knee function and structure. A comprehensive assessment following ACL treatment should aim to provide a complete overview of the treatment result, optimally including the various aspects of outcome categories. For most research questions, a minimum follow-up of 2 years with an optimal follow-up rate of 80% is necessary to achieve a comprehensive assessment. This should include clinical examination, any sustained re-injuries, validated knee-specific PROs and Health-Related Quality of Life questionnaires. In the mid- to long-term follow-up, the presence of osteoarthritis should be evaluated.
Conclusion: This consensus paper provides practical guidelines for how the aforementioned entities of outcomes should be reported and suggests the preferred tools for a reliable and valid assessment of outcome after ACL treatment.
Level of evidence: V.
Keywords: ACL; Anterior cruciate ligament; Consensus statement; Outcome; Reconstruction.
Conflict of interest statement
Dr. Karlsson is the Editor-in-Chief of KSSTA. Dr. Irrgang reports grants from American Orthopaedic Society for Sports Medicine, outside the submitted work. Dr. Spindler reports grants from NIH/NIAMS R01 AR053684, grants from NIH/NIAMS R01 AR074131, grants from NIH/NIAMS R01 AR075422-01, other from NFL, other from Service Excellence, other from Mitek, other from Flexion Therapeutics, other from Samumed, other from Novopeds, other from nPhase, outside the submitted work. Dr. Musahl reports educational grants, consulting fees, and speaking fees from Smith & Nephew and educational grants from Arthrex. Dr. Ayeni reports Speakers Bureau from Conmed, honoraria from DJO, outside the submitted work. Dr. Dye reports personal fees from Zimmer Biomet, outside the submitted work. Dr. Fu reports educational support and hospitality payments from Smith & Nephew, outside the submitted work. Dr. Getgood reports grants and personal fees from Smith & Nephew, grants and personal fees from Ossur, personal fees from Graymont, personal fees from Olympus, outside the submitted work. Dr. Kuroda reports grants and personal fees from Smith & Nephew KK, grants and personal fees from Jimmer Biomet, grants from Stryker Japan KK, grants and personal fees from Johnson & Johnson KK, personal fees from Medacta International, personal fees from Arthrex, Inc, personal fees from Japan Tissue Engineering Co., Ltd, personal fees from Hirosaki Life Science Innovation, Inc, personal fees from Arthrex Japan G.K., outside the submitted work. Dr. Lesniak reports personal fees and other from Wolters Kluwer Health - Lippincott Williams & Wilkins, outside the submitted work. Dr. Marx reports personal fees from Journal of Bone and Joint Surgery, personal fees from Journal of Bone and Joint Surgery Evidence-Based Orthopedics, personal fees from MEND Nutrition Inc., personal fees from Springer & Demos Health, outside the submitted work. Dr. Pinczewski reports personal fees from Australian Biotechnologies, grants from Australian Orthopaedic Association, grants from Friends of the Mater Foundation, personal fees from Signature Orthopaedics, grants and personal fees from Smith and Newphew, outside the submitted work. In addition, Dr. Pinczewski has a patent Surgical screw pending to Hip Developments Pty Ltd., a patent Process and article for knee reconstruction pending to Smith & Nephew, Inc., a patent Method of arthroplasty on a knee joint and apparatus for use in same pending to Smith & Nephew, Inc., and a patent Manually operated medical pump pending to SURGICAL APPS Pty Ltd. Dr. Ranawat reports other from Enhatch, other from Conformis, other from Stryker, other from Smith and Nephew, other from Arthrex, Inc., from Anika, from Bodycad, outside the submitted work. Dr. Reider reports other from Smith and Nephew, personal fees from Elsevier, other from Merck, other from Johnson and Johnson, outside the submitted work. Dr. Wolf reports personal fees from ConMed, from null, outside the submitted work. Dr Zheng is the founder of Orthocell Ltd and hold share of Orthocell that is not directly related to the work.
Figures
References
-
- Ajuied A, Wong F, Smith C, Norris M, Earnshaw P, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242–2252. - PubMed
-
- Andernord D, Karlsson J, Musahl V, Bhandari M, Fu FH, et al. Timing of surgery of the anterior cruciate ligament. Arthroscopy. 2013;29(11):1863–1871. - PubMed
-
- Anderson JP, Kaplan RM, Berry CC, Bush JW, Rumbaut RG. Interday reliability of function assessment for a health status measure. The Quality of Well-Being scale. Med Care. 1989;27(11):1076–1083. - PubMed
-
- Andersson-Molina H, Karlsson H, Rockborn P. Arthroscopic partial and total meniscectomy: a long-term follow-up study with matched controls. Arthroscopy. 2002;18(2):183–189. - PubMed
-
- Andersson D, Samuelsson K, Karlsson J. Treatment of anterior cruciate ligament injuries with special reference to surgical technique and rehabilitation: an assessment of randomized controlled trials. Arthroscopy. 2009;25(6):653–685. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous