Over- and undersensing-pitfalls of arrhythmia detection with implantable devices and wearables
- PMID: 32767089
- PMCID: PMC7412442
- DOI: 10.1007/s00399-020-00710-x
Over- and undersensing-pitfalls of arrhythmia detection with implantable devices and wearables
Abstract
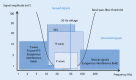
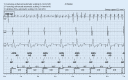
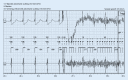
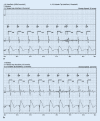
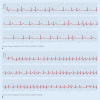
Cardiac implantable electronic devices (CIEDs) are a cornerstone of arrhythmia and heart failure detection as well as management. In recent years new kinds of devices have emerged which can be used subcutaneously or worn on the skin. In particular for large-scale arrhythmia monitoring, small, unobtrusive gadgets seem positioned to upend paradigms and care delivery. However, the performance of CIEDs and wearables is only as good as their sensing and detection capacities. Whether for pacing, defibrillation or diagnostic monitoring, the device must be able to process and filter the sensed signal to reduce noise and to exclude irrelevant physiological signals. The demands on sensing and detection quality will differ depending on how the information is applied. With a pacemaker or implantable cardioverter/defibrillator, withheld or erroneous therapy can have severe consequences and accurate and reliable detection of cardiac function is crucial. Monitoring devices are usually used in risk assessment and management, with greater tolerance for isolated artefacts or lower quality of readings. This review discusses sensing and detection and the performance to date by CIEDs as well as subcutaneous and wearable devices.
Kardiale implantierbare elektronische Systeme (CIED) stellen Eckpfeiler in der Diagnostik und Therapie von Herzrhythmusstörungen und Herzinsuffizienz dar. In den letzten Jahren wurden neue Geräte entwickelt, die subkutan implantiert oder als sogenannte Wearables getragen werden können. Insbesondere im permanenten Arrhythmiemonitoring scheinen kleine, unauffällige Geräte das Potenzial zu haben, Paradigmen umzustoßen und die Versorgung zu verändern. Die Leistung von CIED und Wearables ist jedoch nur so gut wie deren Wahrnehmungs- und Detektionseigenschaften. Unabhängig von der Nutzung als Herzschrittmacher, Defibrillator oder Monitoring-Device muss durch spezielle Filter und Algorithmen sichergestellt werden, dass das wahrgenommene intrinsische Signal von Störsignalen oder irrelevanten physiologischen Signalen differenziert werden kann. Die Anforderungen an die Wahrnehmungs- und Detektionsqualität sind davon abhängig, wie die Informationen genutzt werden. Eine durch Oversensing oder Undersensing zurückgehaltene oder falsche Therapie bei Patienten mit Herzschrittmacher oder implantierbarem Kardioverter/Defibrillator kann zu schweren Komplikationen führen, weshalb eine genaue und verlässliche Detektion der Herzfunktion hier kritisch ist. Systeme zum alleinigen Monitoring finden vor allem in der Risikoabschätzung und Therapieoptimierung Verwendung, wobei vereinzelte Artefakte oder eine geringere Messqualität hier tolerabler sind. Die vorliegende Übersicht beschreibt die Wahrnehmungs- und Detektionsfunktion sowie die resultierende Qualität der verfügbaren CIED, subkutanen Systeme und Wearables.
Keywords: Arrhythmia detection; Defibrillator; Over- and undersensing; Pacemaker; Wearables.
Conflict of interest statement
J. Sperzel received speaker fees and institutional research grants from Abbott, Biotronik, Boston Scientific, Impulse Dynamics, Microport and Zoll. C.W. Hamm and A. Hain declare that they have no competing interests.
Figures







References
-
- Auricchio A, Schloss EJ, Kurita T, et al. Low inappropriate shock rates in patients with single- and dual/triple-chamber implantable cardioverter-defibrillators using a novel suite of detection algorithms: PainFree SST trial primary results. Heart Rhythm. 2015;12:926–936. doi: 10.1016/j.hrthm.2015.01.017. - DOI - PubMed
-
- Bagchi C (2020) Telehealth in the era of COVID-19. https://www.eiu.com/n/telehealth-in-the-era-of-covid-19/. Accessed 10 June 2020
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

