X-linked muscular dystrophy in a Labrador Retriever strain: phenotypic and molecular characterisation
- PMID: 32767978
- PMCID: PMC7412789
- DOI: 10.1186/s13395-020-00239-0
X-linked muscular dystrophy in a Labrador Retriever strain: phenotypic and molecular characterisation
Abstract
Background: Canine models of Duchenne muscular dystrophy (DMD) are a valuable tool to evaluate potential therapies because they faithfully reproduce the human disease. Several cases of dystrophinopathies have been described in canines, but the Golden Retriever muscular dystrophy (GRMD) model remains the most used in preclinical studies. Here, we report a new spontaneous dystrophinopathy in a Labrador Retriever strain, named Labrador Retriever muscular dystrophy (LRMD).
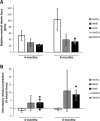
Methods: A colony of LRMD dogs was established from spontaneous cases. Fourteen LRMD dogs were followed-up and compared to the GRMD standard using several functional tests. The disease causing mutation was studied by several molecular techniques and identified using RNA-sequencing.
Results: The main clinical features of the GRMD disease were found in LRMD dogs; the functional tests provided data roughly overlapping with those measured in GRMD dogs, with similar inter-individual heterogeneity. The LRMD causal mutation was shown to be a 2.2-Mb inversion disrupting the DMD gene within intron 20 and involving the TMEM47 gene. In skeletal muscle, the Dp71 isoform was ectopically expressed, probably as a consequence of the mutation. We found no evidence of polymorphism in either of the two described modifier genes LTBP4 and Jagged1. No differences were found in Pitpna mRNA expression levels that would explain the inter-individual variability.
Conclusions: This study provides a full comparative description of a new spontaneous canine model of dystrophinopathy, found to be phenotypically equivalent to the GRMD model. We report a novel large DNA mutation within the DMD gene and provide evidence that LRMD is a relevant model to pinpoint additional DMD modifier genes.
Keywords: Animal model; Canine; DMD; Dog; Dp71; Dystrophin; Inversion; LRMD; Neuromuscular disorders.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. - PubMed
-
- Valentine BA, Cooper BJ, de LA, O'Quinn R, Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J.Neurol.Sci. 1988;88:69-81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

