Acetazolamide for OSA and Central Sleep Apnea: A Comprehensive Systematic Review and Meta-Analysis
- PMID: 32768459
- PMCID: PMC7768933
- DOI: 10.1016/j.chest.2020.06.078
Acetazolamide for OSA and Central Sleep Apnea: A Comprehensive Systematic Review and Meta-Analysis
Abstract
Background: Therapy options for OSA and central sleep apnea (CSA) are limited, thus many patients remain untreated. Clinically, acetazolamide is sometimes used for CSA; however, given overlapping pathophysiologic properties of OSA and CSA, we hypothesized that acetazolamide is equally effective for both types. Prior reviews focused on specific subtypes of sleep apnea, study designs, and languages, thus including few studies (typically ≤3) limiting insights.
Research question: How efficacious is acetazolamide for sleep apnea, and is its effect modified by sleep apnea type or acetazolamide dose?
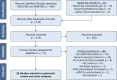
Study design and methods: We queried MEDLINE, EMBASE, and ClinicalTrials.gov from inception until March 11, 2019. Any study in which adults with OSA/CSA received oral acetazolamide vs no acetazolamide (control) that reported sleep apnea-related outcomes was eligible, independent of study design or language. Two reviewers independently assessed eligibility and abstracted data. Primary outcomes were apnea-hypopnea index (AHI) and oxygen saturation nadir. Quality of evidence (QoE) was rated with the use of Grades of Recommendation Assessment, Development and Evaluation methods.
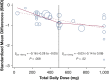
Results: We included 28 studies (13 OSA/15 CSA; NSubjects,Acetazolamide = 542; NSubjects,Control = 553) that enabled meta-analyses for 24 outcomes. Acetazolamide doses ranged from 36 to 1000 mg/d and treatment duration from 1 to 90 d (median, 6 d). Overall, acetazolamide vs control lowered the AHI by -0.7 effect sizes (95% CI, -0.83 to -0.58; I2 = 0%; moderate QoE) that corresponded to a reduction of 37.7% (95% CI, -44.7 to -31.3) or 13.8/h (95% CI, -16.3 to -11.4; AHIControl = 36.5/h). The AHI reduction was similar in OSA vs CSA, but significantly greater with higher doses (at least up to 500 mg/d). Furthermore, acetazolamide improved oxygen saturation nadir by +4.4% (95% CI, 2.3 to 6.5; I2 = 63%; no evidence of effect modification; very low QoE) and several secondary outcomes that included sleep quality measures and BP (mostly low QoE).
Interpretation: Short-term acetazolamide improved both OSA and CSA. Rigorous studies with long-term follow up are warranted to assess Acetazolamide's value for the chronic treatment of patients with sleep apnea.
Clinical trial registration: PROSPERO (CRD42019147504).
Keywords: acetazolamide; apnea-hypopnea index; sleep apnea.
Copyright © 2020 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures