Recent advances in siRNA delivery mediated by lipid-based nanoparticles
- PMID: 32768564
- PMCID: PMC7406478
- DOI: 10.1016/j.addr.2020.07.022
Recent advances in siRNA delivery mediated by lipid-based nanoparticles
Abstract
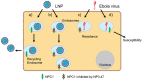
Small interfering RNA (siRNA) has been expected to be a unique pharmaceutic for the treatment of broad-spectrum intractable diseases. However, its unfavorable properties such as easy degradation in the blood and negative-charge density are still a formidable barrier for clinical use. For disruption of this barrier, siRNA delivery technology has been significantly advanced in the past two decades. The approval of Patisiran (ONPATTRO™) for the treatment of transthyretin-mediated amyloidosis, the first approved siRNA drug, is a most important milestone. Since lipid-based nanoparticles (LNPs) are used in Patisiran, LNP-based siRNA delivery is now of significant interest for the development of the next siRNA formulation. In this review, we describe the design of LNPs for the improvement of siRNA properties, bioavailability, and pharmacokinetics. Recently, a number of siRNA-encapsulated LNPs were reported for the treatment of intractable diseases such as cancer, viral infection, inflammatory neurological disorder, and genetic diseases. We believe that these contributions address and will promote the development of an effective LNP-based siRNA delivery system and siRNA formulation.
Keywords: Lipid nanoparticles; pH-responsive ionizable lipid; siRNA.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

