Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2
- PMID: 32769024
- PMCID: PMC7396134
- DOI: 10.1016/j.jcv.2020.104572
Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2
Abstract
Background: The emergence of SARS-CoV-2 has led to the development of serological assays that could aid in an understanding of the burden of COVID-19 disease. Many available tests lack rigorous evaluation and therefore results may be misleading.
Objectives: The aim of this study was to assess the performance of a novel multiplexed immunoassay for the simultaneous detection of antibodies against SARS-CoV-2 trimeric spike (S), spike receptor binding domain (RBD), spike N terminal domain and nucleocapsid antigen and a novel pseudo-neutralisation assay.
Methods: A multiplexed solid-phase chemiluminescence assay (Meso Scale Discovery) was evaluated for the simultaneous detection of IgG binding to four SARS-CoV-2 antigens and the quantification of antibody-induced ACE-2 binding inhibition (pseudo-neutralisation assay). Sensitivity was evaluated with a total of 196 COVID-19 serum samples (169 confirmed PCR positive and 27 anti-nucleocapsid IgG positive) from individuals with mild symptomatic or asymptomatic disease. Specificity was evaluated with 194 control serum samples collected from adults prior to December 2019.
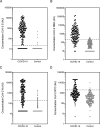
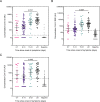
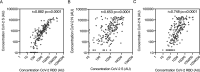
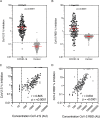
Results: The specificity and sensitivity of the binding IgG assay was highest for S protein with a specificity of 97.4 % and sensitivity of 96.2 % for samples taken 14 days and 97.9 % for samples taken 21 days following the onset of symptoms. IgG concentration to S and RBD correlated strongly with percentage inhibition measured by the pseudo-neutralisation assay.
Conclusion: Excellent sensitivity for IgG detection was obtained over 14 days since onset of symptoms for three SARS-CoV-2 antigens (S, RBD and N) in this multiplexed assay which can also measure antibody functionality.
Keywords: Covid19; Immunoassays; Immunology; SARS-CoV-2.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures

References
-
- World Health Organisation . 2020. Coronavrius Disease 2019 (COVID-19) Situation Report - 51.
-
- Salazar E., Kuchipudi S.V., Christensen P.A., Eagar T.N., Yi X., Zhao P., Jin Z., Long S.W., Olsen R.J., Chen J., Castillo B., Leveque C., Towers D.M., Lavinder J., Gollihar J.D., Cardona J., Ippolito G.C., Nissly R.H., Bird I.M., Greenawalt D., Rossi R.M., Gontu A., Srinivasan S., Poojary I.B., Cattadori I.M., Hudson P.J., Joselyn N., Prugar L., Huie K., Herbert A., Bernard D.W., Dye J., Kapur V., Musser J.M. Relationship between anti-spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. bioRxivorg. 2020 doi: 10.1101/2020.06.08.138990. - DOI - PMC - PubMed
-
- To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S., Lau D.P., Choi C.Y., Chen L.L., Chan W.M., Chan K.H., Ip J.D., Ng A.C., Poon R.W., Luo C.T., Cheng V.C., Chan J.M.F., Hung I.F., Chen Z., Chen H., Yuen K.Y. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–574. - PMC - PubMed
-
- Cheng M.P., Yansouni C.P., Basta N.E., Desjardins M., Kanjilal S., Paquette K., Caya C., Semret M., Quach C., Libman M., Mazzola L., Sacks J.A., Dittrich S., Papenburg J. Serodiagnostics for severe acute respiratory syndrome-related Coronavirus-2: a narrative review. Ann. Intern. Med. 2020 doi: 10.7326/M20-2854. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

