Effect of allosteric inhibition of non-muscle myosin 2 on its intracellular diffusion
- PMID: 32769996
- PMCID: PMC7415145
- DOI: 10.1038/s41598-020-69853-8
Effect of allosteric inhibition of non-muscle myosin 2 on its intracellular diffusion
Abstract
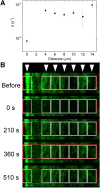
Subcellular dynamics of non-muscle myosin 2 (NM2) is crucial for a broad-array of cellular functions. To unveil mechanisms of NM2 pharmacological control, we determined how the dynamics of NM2 diffusion is affected by NM2's allosteric inhibitors, i.e. blebbistatin derivatives, as compared to Y-27632 inhibiting ROCK, NM2's upstream regulator. We found that NM2 diffusion is markedly faster in central fibers than in peripheral stress fibers. Y-27632 accelerated NM2 diffusion in both peripheral and central fibers, whereas in peripheral fibers blebbistatin derivatives slightly accelerated NM2 diffusion at low, but markedly slowed it at high inhibitor concentrations. In contrast, rapid NM2 diffusion in central fibers was unaffected by direct NM2 inhibition. Using our optopharmacological tool, Molecular Tattoo, sub-effective concentrations of a photo-crosslinkable blebbistatin derivative were increased to effective levels in a small, irradiated area of peripheral fibers. These findings suggest that direct allosteric inhibition affects the diffusion profile of NM2 in a markedly different manner compared to the disruption of the upstream control of NM2. The pharmacological action of myosin inhibitors is channeled through autonomous molecular processes and might be affected by the load acting on the NM2 proteins.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

