Structural and functional connectivity from the dorsomedial hypothalamus to the ventral medulla as a chronological amplifier of sympathetic outflow
- PMID: 32770006
- PMCID: PMC7414200
- DOI: 10.1038/s41598-020-70234-4
Structural and functional connectivity from the dorsomedial hypothalamus to the ventral medulla as a chronological amplifier of sympathetic outflow
Abstract
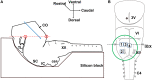
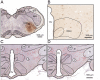
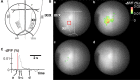
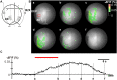
Psychological stress activates the hypothalamus, augments the sympathetic nervous output, and elevates blood pressure via excitation of the ventral medullary cardiovascular regions. However, anatomical and functional connectivity from the hypothalamus to the ventral medullary cardiovascular regions has not been fully elucidated. We investigated this issue by tract-tracing and functional imaging in rats. Retrograde tracing revealed the rostral ventrolateral medulla was innervated by neurons in the ipsilateral dorsomedial hypothalamus (DMH). Anterograde tracing showed DMH neurons projected to the ventral medullary cardiovascular regions with axon terminals in contiguity with tyrosine hydroxylase-immunoreactive neurons. By voltage-sensitive dye imaging, dynamics of ventral medullary activation evoked by electrical stimulation of the DMH were analyzed in the diencephalon-lower brainstem-spinal cord preparation of rats. Although the activation of the ventral medulla induced by single pulse stimulation of the DMH was brief, tetanic stimulation caused activation of the DMH sustained into the post-stimulus phase, resulting in delayed recovery. We suggest that prolonged excitation of the DMH, which is triggered by tetanic electrical stimulation and could also be triggered by psychological stress in a real life, induces further prolonged excitation of the medullary cardiovascular networks, and could contribute to the pathological elevation of blood pressure. The connectivity from the DMH to the medullary cardiovascular networks serves as a chronological amplifier of stress-induced sympathetic excitation. This notion will be the anatomical and pathophysiological basis to understand the mechanisms of stress-induced sustained augmentation of sympathetic activity.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus.Neuroscience. 2004;126(1):229-40. doi: 10.1016/j.neuroscience.2004.03.013. Neuroscience. 2004. PMID: 15145088
-
Differential control of cardiac and sympathetic vasomotor activity from the dorsomedial hypothalamus.Clin Exp Pharmacol Physiol. 2006 Dec;33(12):1265-8. doi: 10.1111/j.1440-1681.2006.04522.x. Clin Exp Pharmacol Physiol. 2006. PMID: 17184513 Review.
-
Differential responses of sympathetic premotor neurons in the rostral ventrolateral medulla to stimulation of the dorsomedial hypothalamus in rabbits.Brain Res. 2010 Oct 14;1356:44-53. doi: 10.1016/j.brainres.2010.08.024. Epub 2010 Aug 13. Brain Res. 2010. PMID: 20713029
-
Role of 5-HT(1A) receptors in the lower brainstem on the cardiovascular response to dorsomedial hypothalamus activation.Auton Neurosci. 2008 Nov 3;142(1-2):71-6. doi: 10.1016/j.autneu.2008.06.004. Epub 2008 Jul 29. Auton Neurosci. 2008. PMID: 18667366
-
The role of the RVLM neurons in the viscero-sympathetic reflex: a mini review.Auton Neurosci. 2008 Nov 3;142(1-2):17-9. doi: 10.1016/j.autneu.2008.03.007. Epub 2008 May 23. Auton Neurosci. 2008. PMID: 18457999 Review.
Cited by
-
Central stress pathways in the development of cardiovascular disease.Clin Auton Res. 2024 Feb;34(1):99-116. doi: 10.1007/s10286-023-01008-x. Epub 2023 Dec 17. Clin Auton Res. 2024. PMID: 38104300 Review.
-
Persistence of post-stress blood pressure elevation requires activation of astrocytes.Sci Rep. 2024 Oct 3;14(1):22984. doi: 10.1038/s41598-024-73345-4. Sci Rep. 2024. PMID: 39363030 Free PMC article.
-
Cardiac-Sympathetic Contractility and Neural Alpha-Band Power: Cross-Modal Collaboration during Approach-Avoidance Conflict.J Neurosci. 2024 Oct 9;44(41):e2008232024. doi: 10.1523/JNEUROSCI.2008-23.2024. J Neurosci. 2024. PMID: 39214705 Free PMC article.
-
The Central Nervous Mechanism of Stress-Promoting Cancer Progression.Int J Mol Sci. 2022 Oct 21;23(20):12653. doi: 10.3390/ijms232012653. Int J Mol Sci. 2022. PMID: 36293510 Free PMC article. Review.
-
Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats.Int J Mol Sci. 2023 Aug 19;24(16):12985. doi: 10.3390/ijms241612985. Int J Mol Sci. 2023. PMID: 37629166 Free PMC article.
References
-
- Sah P, Faber ES, Lopez de Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. - PubMed
-
- de Almeida DO, Ferreira HS, Pereira LB, Fregoneze JB. Hypertensive response to stress: the role of histaminergic H1 and H2 receptors in the medial amygdala. Physiol. Behav. 2015;144:95–102. - PubMed
-
- Kataoka N, Shima Y, Nakajima K, Nakamura K. A central master driver of psychosocial stress responses in the rat. Science. 2020;367:1105–1112. - PubMed
-
- DiMicco JA, Stotz-potter EH, Monroe AJ, Morin SM. Role of the dorsomedial hypothalamus in the cardiovascular response to stress. Clin. Exp. Pharmacol. Physiol. 1996;23:171–176. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

