Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation
- PMID: 32770173
- PMCID: PMC8027053
- DOI: 10.1038/s41401-020-0490-7
Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation
Abstract
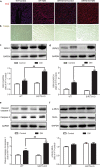
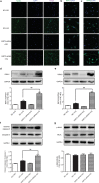
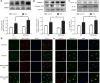
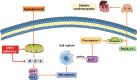
Sirtuin 3 (SIRT3) is a potential therapeutic target for cardiovascular, metabolic, and other aging-related diseases. In this study, we investigated the role of SIRT3 in diabetic cardiomyopathy (DCM). Mice were injected with streptozotocin (STZ, 60 mg/kg, ip) to induce diabetes mellitus. Our proteomics analysis revealed that SIRT3 expression in the myocardium of diabetic mice was lower than that of control mice, as subsequently confirmed by real-time PCR and Western blotting. To explore the role of SIRT3 in DCM, SIRT3-knockout mice and 129S1/SvImJ wild-type mice were injected with STZ. We found that diabetic mice with SIRT3 deficiency exhibited aggravated cardiac dysfunction, increased lactate dehydrogenase (LDH) level in the serum, decreased adenosine triphosphate (ATP) level in the myocardium, exacerbated myocardial injury, and promoted myocardial reactive oxygen species (ROS) accumulation. Neonatal rat cardiomyocytes were transfected with SIRT3 siRNA, then exposed to high glucose (HG, 25.5 mM). We found that downregulation of SIRT3 further increased LDH release, decreased ATP level, suppressed the mitochondrial membrane potential, and elevated oxidative stress in HG-treated cardiomyocytes. SIRT3 deficiency further raised expression of necroptosis-related proteins including receptor-interacting protein kinase 1 (RIPK1), RIPK3, and cleaved caspase 3, and upregulated the expression of inflammation-related proteins including NLR family pyrin domain-containing protein 3 (NLRP3), caspase 1 p20, and interleukin-1β both in vitro and in vivo. Collectively, SIRT3 deficiency aggravated hyperglycemia-induced mitochondrial damage, increased ROS accumulation, promoted necroptosis, possibly activated the NLRP3 inflammasome, and ultimately exacerbated DCM in the mice. These results suggest that SIRT3 can be a molecular intervention target for the prevention and treatment of DCM.
Keywords: NLRP3; RIPK3; ROS; Sirtuin 3; diabetic cardiomyopathy; mitochondria; necroptosis.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
SIRT3 deficiency delays diabetic skin wound healing via oxidative stress and necroptosis enhancement.J Cell Mol Med. 2020 Apr;24(8):4415-4427. doi: 10.1111/jcmm.15100. Epub 2020 Mar 2. J Cell Mol Med. 2020. PMID: 32119761 Free PMC article.
-
NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model.PLoS One. 2014 Aug 19;9(8):e104771. doi: 10.1371/journal.pone.0104771. eCollection 2014. PLoS One. 2014. PMID: 25136835 Free PMC article.
-
Neuraminidase 1 deficiency attenuates cardiac dysfunction, oxidative stress, fibrosis, inflammatory via AMPK-SIRT3 pathway in diabetic cardiomyopathy mice.Int J Biol Sci. 2022 Jan 1;18(2):826-840. doi: 10.7150/ijbs.65938. eCollection 2022. Int J Biol Sci. 2022. PMID: 35002528 Free PMC article.
-
Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy.Biochim Biophys Acta Mol Basis Dis. 2017 Aug;1863(8):1973-1983. doi: 10.1016/j.bbadis.2016.10.021. Epub 2016 Oct 26. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27794418 Review.
-
NLRP3 Inflammasome in Diabetic Cardiomyopathy and Exercise Intervention.Int J Mol Sci. 2021 Dec 8;22(24):13228. doi: 10.3390/ijms222413228. Int J Mol Sci. 2021. PMID: 34948026 Free PMC article. Review.
Cited by
-
An update on the regulatory mechanisms of NLRP3 inflammasome activation.Cell Mol Immunol. 2021 May;18(5):1141-1160. doi: 10.1038/s41423-021-00670-3. Epub 2021 Apr 13. Cell Mol Immunol. 2021. PMID: 33850310 Free PMC article. Review.
-
Investigating EGF and PAG1 as necroptosis-related biomarkers for diabetic nephropathy: an in silico and in vitro validation study.Aging (Albany NY). 2023 Nov 20;15(22):13176-13193. doi: 10.18632/aging.205233. Epub 2023 Nov 20. Aging (Albany NY). 2023. PMID: 37988198 Free PMC article.
-
The Role of H2S Regulating NLRP3 Inflammasome in Diabetes.Int J Mol Sci. 2022 Apr 27;23(9):4818. doi: 10.3390/ijms23094818. Int J Mol Sci. 2022. PMID: 35563208 Free PMC article. Review.
-
The role of hydrogen sulfide in the regulation of necroptosis across various pathological processes.Mol Cell Biochem. 2025 Apr;480(4):1999-2013. doi: 10.1007/s11010-024-05090-1. Epub 2024 Aug 14. Mol Cell Biochem. 2025. PMID: 39138751 Review.
-
Mitochondrial quality control in health and cardiovascular diseases.Front Cell Dev Biol. 2023 Nov 6;11:1290046. doi: 10.3389/fcell.2023.1290046. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38020895 Free PMC article. Review.
References
-
- Al Hroob AM, Abukhalil MH, Hussein OE, Mahmoud AM. Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate. Biomed Pharmacother. 2019;109:2155–72. - PubMed
-
- Alonso N, Moliner P, Mauricio D. Pathogenesis, clinical features and treatment of diabetic cardiomyopathy. Adv Exp Med Biol. 2018;1067:197–217. - PubMed
-
- Cao H, Chen T, Shi Y. Glycation of human serum albumin in diabetes: impacts on the structure and function. Curr Med Chem. 2015;22:4–13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

