Lessons learned from early compassionate use of convalescent plasma on critically ill patients with Covid-19
- PMID: 32770691
- PMCID: PMC7436937
- DOI: 10.1111/trf.15975
Lessons learned from early compassionate use of convalescent plasma on critically ill patients with Covid-19
Abstract
Background: The management of critically ill patients with coronavirus disease 2019 (COVID-19), caused by a new human virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is challenging. Recently, there have been several reports with inconsistent results after treatment with convalescent plasma (CP) on critically ill patients with COVID-19, which was produced with a neutralizing antibody titer and tested in a P3 or P4 laboratory. However, due to the limitation of the conditions on mass production of plasma, most producers hardly had the capability to isolate the neutralizing antibody. Here, we report the clinical courses of three critically ill patients with COVID-19 receiving CP treatments by total immunoglobulin G (IgG) titer collection.
Methods: Three patients with COVID-19 in this study were laboratory confirmed to be positive for SARS-CoV-2, with radiographic and clinical features of pneumonia. CP was collected by total IgG titer of 160 (range, 200-225 mL), and patients were transfused between 20 and 30 days after disease onset at the critical illness stage as a trial in addition to standard care. The clinical courses of these patients, including laboratory results and pulmonary functional and image studies after receiving convalescent plasma infusions, were reviewed.
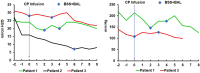
Results: No therapeutic effect of CP was observed in any of the patients; instead, all three patients deteriorated and required extracorporeal membrane oxygenation treatment. A potential cytokine storm 4 hours after infusion of CP in Patient 2 was observed. No more patients were put on the trial of CP transfusion.
Conclusions: We recommend extreme caution in using CP in critically ill patients more than 2 weeks after the onset of COVID-19 pneumonia.
© 2020 AABB.
Conflict of interest statement
None of the material has been published or is under consideration for publication elsewhere. We have no conflict of interest that might influence our results or their interpretation.
Figures
References
-
- Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2:695–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL066987/HL/NHLBI NIH HHS/United States
- This study was supported Health and Family Planning Commission of Wuhan Municipality (WX18A07); by the National Institutes of Health (NIH) T32 HL066987 (M.L.); by the Singapore Ministry of Health's National Medical Research Council under its Singapore Translational Research (STaR) Investigator Award MOH-STaR18nov-0002 (D.G.T.); as well as NIH/NHLBI Grant P01HL095489 and Xiu Research Fund (L.C.)
- This study was supported Health and Family Planning Commission of Wuhan Municipality (WX18A07); by the National Institutes of Health (NIH) T32 HL066987 (M.L.); by the Singapore Ministry of Health's National Medical Research Council under its Singapore Translational Research (STaR) Investigator Award MOH-STaR18nov-0002 (D.G.T.); as well as NIH/NHLBI Grant P01HL095489 and Xiu Research Fund (L.C.).
- P01 HL131477/HL/NHLBI NIH HHS/United States
- R35 CA197697/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

