Epigenetic Changes Associated With Anthracycline-Induced Cardiotoxicity
- PMID: 32770710
- PMCID: PMC7877852
- DOI: 10.1111/cts.12857
Epigenetic Changes Associated With Anthracycline-Induced Cardiotoxicity
Abstract
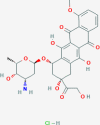
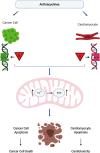
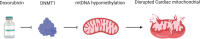
Advances in cancer treatment have significantly improved the survival of patients with cancer, but, unfortunately, many of these treatments also have long-term complications. Cancer treatment-related cardiotoxicities are becoming a significant clinical problem that a new discipline, Cardio-Oncology, was established to advance the cardiovascular care of patients with growing cancer populations. Anthracyclines are a class of chemotherapeutic agents used to treat many cancers in adults and children. Their clinical use is limited by anthracycline-induced cardiotoxicity (AIC), which can lead to heart failure. Early-onset cardiotoxicity appears within a year of treatment, whereas late-onset cardiotoxicity occurs > 1 year and even up to decades after treatment completion. The pathophysiology of AIC was hypothesized to be caused by generation of reactive oxygen species that lead to lipid peroxidation, defective mitochondrial biogenesis, and DNA damage of the cardiomyocytes. The accumulation of anthracycline metabolites was also proposed to cause mitochondrial damage and the induction of cardiac cell apoptosis, which induces arrhythmias, contractile dysfunction, and cardiomyocyte death. This paper will provide a general overview of cardiotoxicity focusing on the effect of anthracyclines and their epigenetic molecular mechanisms on cardiotoxicity.
© 2020 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work. As an Editor‐in‐Training for
Figures
References
-
- World Health Organization . WHO Model List of Essential Medicines. 1–43 (World Health Organization, Austria, 2013). <https://www.who.int/medicines/publications/essentialmedicines/18th_EML.pdf>.
-
- Siegel, R.L. , Miller, K.D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

