Functional glutamate transporters are expressed in the carotid chemoreceptor
- PMID: 32771007
- PMCID: PMC7414757
- DOI: 10.1186/s12931-020-01468-z
Functional glutamate transporters are expressed in the carotid chemoreceptor
Abstract
Background: The carotid body (CB) plays a critical role in cyclic intermittent hypoxia (CIH)-induced chemosensitivity; however, the underlying mechanism remains uncertain. We have demonstrated the presence of multiple inotropic glutamate receptors (iGluRs) in CB, and that CIH exposure alters the level of some iGluRs in CB. This result implicates glutamatergic signaling in the CB response to hypoxia. The glutamatergic neurotransmission is not only dependent on glutamate and glutamate receptors, but is also dependent on glutamate transporters, including vesicular glutamate transporters (VGluTs) and excitatory amino acid transporters (EAATs). Here, we have further assessed the expression and distribution of VGluTs and EAATs in human and rat CB and the effect of CIH exposure on glutamate transporters expression.
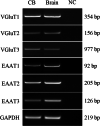
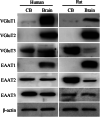
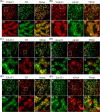
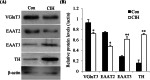
Methods: The mRNA of VGluTs and EAATs in the human CB were detected by RT-PCR. The protein expression of VGluTs and EAATs in the human and rat CB were detected by Western blot. The distribution of VGluT3, EAAT2 and EAAT3 were observed by immunohistochemistry staining and immunofluorescence staining. Male Sprague-Dawley (SD) rats were exposed to CIH (FIO2 10-21%, 3 min/3 min for 8 h per day) for 2 weeks. The unpaired Student's t-test was performed.
Results: Here, we report on the presence of mRNAs for VGluT1-3 and EAAT1-3 in human CB, which is consistent with our previous results in rat CB. The proteins of VGluT1 and 3, EAAT2 and 3, but not VGluT2 and EAAT1, were detected with diverse levels in human and rat CB. Immunostaining showed that VGluT3, the major type of VGluTs in CB, was co-localized with tyrosine hydroxylase (TH) in type I cells. EAAT2 and EAAT3 were distributed not only in type I cells, but also in glial fibrillary acidic protein (GFAP) positive type II cells. Moreover, we found that exposure of SD rats to CIH enhanced the protein level of EAAT3 as well as TH, but attenuated the levels of VGluT3 and EAAT2 in CB.
Conclusions: Our study suggests that glutamate transporters are expressed in the CB, and that glutamate transporters may contribute to glutamatergic signaling-dependent carotid chemoreflex to CIH.
Keywords: Carotid body; Cyclic intermittent hypoxia; Excitatory amino acid transporter; Glutamate; Vesicular glutamate transporter.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study JAMA. 2000;283(14):1829–1836. - PubMed
-
- Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, Lorenzi-Filho G. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105(8):1135–1139. - PubMed
-
- Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of systemic blood pressure in rats. Hypertension. 1992;19(6 Pt 1):555–561. - PubMed
-
- Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992;20(5):612–619. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

