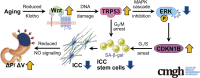
Wnt-induced, TRP53-mediated Cell Cycle Arrest of Precursors Underlies Interstitial Cell of Cajal Depletion During Aging
- PMID: 32771388
- PMCID: PMC7672319
- DOI: 10.1016/j.jcmgh.2020.07.011
Wnt-induced, TRP53-mediated Cell Cycle Arrest of Precursors Underlies Interstitial Cell of Cajal Depletion During Aging
Abstract
Background & aims: Gastric dysfunction in the elderly may cause reduced food intake, frailty, and increased mortality. The pacemaker and neuromodulator cells interstitial cells of Cajal (ICC) decline with age in humans, and their loss contributes to gastric dysfunction in progeric klotho mice hypomorphic for the anti-aging Klotho protein. The mechanisms of ICC depletion remain unclear. Klotho attenuates Wnt (wingless-type MMTV integration site) signaling. Here, we examined whether unopposed Wnt signaling could underlie aging-associated ICC loss by up-regulating transformation related protein TRP53 in ICC stem cells (ICC-SC).
Methods: Mice aged 1-107 weeks, klotho mice, APCΔ468 mice with overactive Wnt signaling, mouse ICC-SC, and human gastric smooth muscles were studied by RNA sequencing, reverse transcription-polymerase chain reaction, immunoblots, immunofluorescence, histochemistry, flow cytometry, and methyltetrazolium, ethynyl/bromodeoxyuridine incorporation, and ex-vivo gastric compliance assays. Cells were manipulated pharmacologically and by gene overexpression and RNA interference.
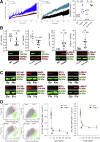
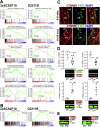
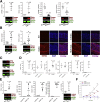
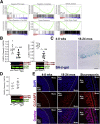
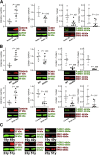
Results: The klotho and aged mice showed similar ICC loss and impaired gastric compliance. ICC-SC decline preceded ICC depletion. Canonical Wnt signaling and TRP53 increased in gastric muscles of klotho and aged mice and middle-aged humans. Overstimulated canonical Wnt signaling increased DNA damage response and TRP53 and reduced ICC-SC self-renewal and gastric ICC. TRP53 induction persistently inhibited G1/S and G2/M cell cycle phase transitions without activating apoptosis, autophagy, cellular quiescence, or canonical markers/mediators of senescence. G1/S block reflected increased cyclin-dependent kinase inhibitor 1B and reduced cyclin D1 from reduced extracellular signal-regulated kinase activity.
Conclusions: Increased Wnt signaling causes age-related ICC loss by up-regulating TRP53, which induces persistent ICC-SC cell cycle arrest without up-regulating canonical senescence markers.
Keywords: Compliance; Senescence; Stem Cell.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Cellular and molecular mechanisms underlying aging-related gastric neuromuscular dysfunction.J Smooth Muscle Res. 2025;61:43-50. doi: 10.1540/jsmr.61.43. J Smooth Muscle Res. 2025. PMID: 40204454 Free PMC article. Review.
-
Insulin-Like Growth Factor1 Preserves Gastric Pacemaker Cells and Motor Function in Aging via ERK1/2 Activation.Cell Mol Gastroenterol Hepatol. 2023;16(3):369-383. doi: 10.1016/j.jcmgh.2023.06.002. Epub 2023 Jun 8. Cell Mol Gastroenterol Hepatol. 2023. PMID: 37301443 Free PMC article.
-
Inhibition of EZH2 Reduces Aging-Related Decline in Interstitial Cells of Cajal of the Mouse Stomach.Cell Mol Gastroenterol Hepatol. 2024;18(4):101376. doi: 10.1016/j.jcmgh.2024.101376. Epub 2024 Jul 3. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38969206 Free PMC article.
-
Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice.J Physiol. 2010 Aug 15;588(Pt 16):3101-17. doi: 10.1113/jphysiol.2010.191023. Epub 2010 Jun 25. J Physiol. 2010. PMID: 20581042 Free PMC article.
-
Klotho, stem cells, and aging.Clin Interv Aging. 2015 Aug 4;10:1233-43. doi: 10.2147/CIA.S84978. eCollection 2015. Clin Interv Aging. 2015. PMID: 26346243 Free PMC article. Review.
Cited by
-
The Crucial Role of the Interstitial Cells of Cajal in Neurointestinal Diseases.Biomolecules. 2023 Sep 7;13(9):1358. doi: 10.3390/biom13091358. Biomolecules. 2023. PMID: 37759758 Free PMC article. Review.
-
Cellular Senescence, Inflammation, and Cancer in the Gastrointestinal Tract.Int J Mol Sci. 2023 Jun 6;24(12):9810. doi: 10.3390/ijms24129810. Int J Mol Sci. 2023. PMID: 37372958 Free PMC article. Review.
-
Protocol for gene knockdown using siRNA in organotypic cultures of murine gastric muscle.J Smooth Muscle Res. 2024;60:64-71. doi: 10.1540/jsmr.60.64. J Smooth Muscle Res. 2024. PMID: 39675996 Free PMC article.
-
The human colon: Evidence for degenerative changes during aging and the physiological consequences.Neurogastroenterol Motil. 2025 Aug;37(8):e14848. doi: 10.1111/nmo.14848. Epub 2024 Jun 17. Neurogastroenterol Motil. 2025. PMID: 38887160 Free PMC article. Review.
-
Cellular and molecular mechanisms underlying aging-related gastric neuromuscular dysfunction.J Smooth Muscle Res. 2025;61:43-50. doi: 10.1540/jsmr.61.43. J Smooth Muscle Res. 2025. PMID: 40204454 Free PMC article. Review.
References
-
- Camilleri M., Cowen T., Koch T.R. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008;20:185–196. - PubMed
-
- Bhutto A., Morley J.E. The clinical significance of gastrointestinal changes with aging. Curr Opin Clin Nutr Metab Care. 2008;11:651–660. - PubMed
-
- Salles N. Is stomach spontaneously ageing? pathophysiology of the ageing stomach. Best Pract Res Clin Gastroenterol. 2009;23:805–819. - PubMed
-
- Parker B.A., Chapman I.M. Food intake and ageing: the role of the gut. Mech Ageing Dev. 2004;125:859–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

