Manipulating the Mediator complex to induce naïve pluripotency
- PMID: 32771524
- PMCID: PMC7584500
- DOI: 10.1016/j.yexcr.2020.112215
Manipulating the Mediator complex to induce naïve pluripotency
Abstract
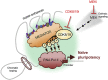
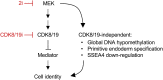
Human naïve pluripotent stem cells (PSCs) represent an optimal homogenous starting point for molecular interventions and differentiation strategies. This is in contrast to the standard primed PSCs which fluctuate in identity and are transcriptionally heterogeneous. However, despite many efforts, the maintenance and expansion of human naïve PSCs remains a challenge. Here, we discuss our recent strategy for the stabilization of human PSC in the naïve state based on the use of a single chemical inhibitor of the related kinases CDK8 and CDK19. These kinases phosphorylate and negatively regulate the multiprotein Mediator complex, which is critical for enhancer-driven recruitment of RNA Pol II. The net effect of CDK8/19 inhibition is a global stimulation of enhancers, which in turn reinforces transcriptional programs including those related to cellular identity. In the case of pluripotent cells, the presence of CDK8/19i efficiently stabilizes the naïve state. Importantly, in contrast to previous chemical methods to induced the naïve state based on the inhibition of the FGF-MEK-ERK pathway, CDK8/19i-naïve human PSCs are chromosomally stable and retain developmental potential after long-term expansion. We suggest this could be related to the fact that CDK8/19 inhibition does not induce DNA demethylation. These principles may apply to other fate decisions.
Keywords: 2i; CDK8; Demethylation; Embryonic stem; MEK; Mediator; Naïve; Phase separation; Pluripotency; Primed; RNA polymerase; Reprogramming; Super-enhancers.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Weinberger L., Ayyash M., Novershtern N., Hanna J.H. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016;17:155–169. - PubMed
-
- Wu J., Izpisua Belmonte J.C. Dynamic pluripotent stem cell states and their applications. Cell Stem Cell. 2015;17:509–525. - PubMed
-
- Wu J., Izpisua Belmonte J.C. Stem cells: a renaissance in human biology Research. Cell. 2016;165:1572–1585. - PubMed
-
- Liu X. Comprehensive characterization of distinct states of human naive pluripotency generated by reprogramming. Nat. Methods. 2017;11:1055–1062. - PubMed
-
- Savatier P., Osteil P., Tam P.P.L. Pluripotency of embryo-derived stem cells from rodents, lagomorphs, and primates: slippery slope, terrace and cliff. Stem Cell Res. 2017;19:104–112. - PubMed

