Exosomes from SIRT1-Overexpressing ADSCs Restore Cardiac Function by Improving Angiogenic Function of EPCs
- PMID: 32771925
- PMCID: PMC7412761
- DOI: 10.1016/j.omtn.2020.07.007
Exosomes from SIRT1-Overexpressing ADSCs Restore Cardiac Function by Improving Angiogenic Function of EPCs
Abstract
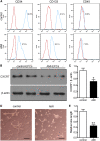
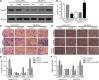
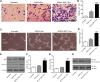
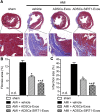
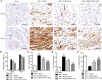
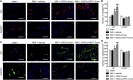
Acute myocardial infarction (AMI) is one of the leading causes of mortality in cardiovascular diseases. The aim of this study was to investigate whether exosomes from Sirtuin 1 (SIRT1)-overexpressing adipose-derived stem cells (ADSCs) had a protective effect on AMI. The expression of C-X-C chemokine receptor type 7 (CXCR7) was significantly downregulated in peripheral blood endothelial progenitor cells (EPCs) from AMI patients (AMI-EPCs) compared with that in healthy donors, which coincided with impaired tube formation. The exosomes from SIRT1 overexpression in ADSCs (ADSCs-SIRT1-Exos) increased the expression of C-X-C motif chemokine 12 (CXCL12) and nuclear factor E2 related factor 2 (Nrf2) in AMI-EPCs, which promoted migration and tube formation of AMI-EPCs, and overexpression of CXCR7 helped AMI-EPCs to restore the function of cell migration and tube formation. Moreover, CXCR7 was downregulated in the myocardium of AMI mice, and knockout of CXCR7 exacerbated AMI-induced impairment of cardiovascular function. Injection of ADSCs-SIRT1-Exos increased the survival and promoted the recovery of myocardial function with reduced infarct size and post-AMI left ventricular remodeling, induced vasculogenesis, and decreased AMI-induced myocardial inflammation. These findings showed that ADSCs-SIRT1-Exos may recruit EPCs to the repair area and that this recruitment may be mediated by Nrf2/CXCL12/CXCR7 signaling.
Keywords: CXCL12; Nrf2; Sirtuin 1; acute myocardial infarction; adipose-tissue-derived stem cells; chemokine receptor CXCR7; exosomes.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
The potential role of circulating exosomes in protecting myocardial injury in acute myocardial infarction via regulating miR-190a-3p/CXCR4/CXCL12 pathway.J Bioenerg Biomembr. 2022 Aug;54(4):175-189. doi: 10.1007/s10863-022-09944-5. Epub 2022 Jul 22. J Bioenerg Biomembr. 2022. PMID: 35867293
-
17β-estradiol promotes recovery after myocardial infarction by enhancing homing and angiogenic capacity of bone marrow-derived endothelial progenitor cells through ERα-SDF-1/CXCR4 crosstalking.Acta Biochim Biophys Sin (Shanghai). 2018 Dec 1;50(12):1247-1256. doi: 10.1093/abbs/gmy127. Acta Biochim Biophys Sin (Shanghai). 2018. PMID: 30371725
-
Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model.Exp Mol Med. 2018 Apr 13;50(4):1-14. doi: 10.1038/s12276-018-0058-5. Exp Mol Med. 2018. PMID: 29651102 Free PMC article.
-
Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review.J Cosmet Dermatol. 2020 Mar;19(3):574-581. doi: 10.1111/jocd.13215. Epub 2019 Nov 21. J Cosmet Dermatol. 2020. PMID: 31755172 Review.
-
The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction.Theranostics. 2021 Jan 1;11(3):1046-1058. doi: 10.7150/thno.53326. eCollection 2021. Theranostics. 2021. PMID: 33391520 Free PMC article. Review.
Cited by
-
Customized Loading of microRNA-126 to Small Extracellular Vesicle-Derived Vehicles Improves Cardiac Function after Myocardial Infarction.ACS Nano. 2023 Oct 24;17(20):19613-19624. doi: 10.1021/acsnano.3c01534. Epub 2023 Sep 16. ACS Nano. 2023. PMID: 37715735 Free PMC article.
-
Intervention effects of traditional Chinese medicine on stem cell therapy of myocardial infarction.Front Pharmacol. 2022 Oct 18;13:1013740. doi: 10.3389/fphar.2022.1013740. eCollection 2022. Front Pharmacol. 2022. PMID: 36330092 Free PMC article. Review.
-
Nrf2 activation: a key mechanism in stem cell exosomes-mediated therapies.Cell Mol Biol Lett. 2024 Mar 2;29(1):30. doi: 10.1186/s11658-024-00551-3. Cell Mol Biol Lett. 2024. PMID: 38431569 Free PMC article. Review.
-
Tailored Extracellular Vesicles: Novel Tool for Tissue Regeneration.Stem Cells Int. 2022 Jul 29;2022:7695078. doi: 10.1155/2022/7695078. eCollection 2022. Stem Cells Int. 2022. PMID: 35915850 Free PMC article. Review.
-
Adipose-Derived Mesenchymal Stem Cells Combined With Extracellular Vesicles May Improve Amyotrophic Lateral Sclerosis.Front Aging Neurosci. 2022 May 18;14:830346. doi: 10.3389/fnagi.2022.830346. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35663577 Free PMC article. Review.
References
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. - PubMed
-
- Lee T.M., Harn H.J., Chiou T.W., Chuang M.H., Chen C.H., Lin P.C., Lin S.Z. Targeting the pathway of GSK-3β/nerve growth factor to attenuate post-infarction arrhythmias by preconditioned adipose-derived stem cells. J. Mol. Cell. Cardiol. 2017;104:17–30. - PubMed
-
- Toma C., Pittenger M.F., Cahill K.S., Byrne B.J., Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. - PubMed
-
- Amado L.C., Saliaris A.P., Schuleri K.H., St John M., Xie J.S., Cattaneo S., Durand D.J., Fitton T., Kuang J.Q., Stewart G. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102:11474–11479. - PMC - PubMed
LinkOut - more resources
Full Text Sources

