Rapamycin Induces an eNOS (Endothelial Nitric Oxide Synthase) Dependent Increase in Brain Collateral Perfusion in Wistar and Spontaneously Hypertensive Rats
- PMID: 32772681
- PMCID: PMC7484020
- DOI: 10.1161/STROKEAHA.120.029781
Rapamycin Induces an eNOS (Endothelial Nitric Oxide Synthase) Dependent Increase in Brain Collateral Perfusion in Wistar and Spontaneously Hypertensive Rats
Abstract
Background and purpose: Rapamycin is a clinically approved mammalian target of rapamycin inhibitor that has been shown to be neuroprotective in animal models of stroke. However, the mechanism of rapamycin-induced neuroprotection is still being explored. Our aims were to determine if rapamycin improved leptomeningeal collateral perfusion, to determine if this is through eNOS (endothelial nitric oxide synthase)-mediated vessel dilation and to determine if rapamycin increases immediate postreperfusion blood flow.
Methods: Wistar and spontaneously hypertensive rats (≈14 weeks old, n=22 and n=15, respectively) were subjected to ischemia by middle cerebral artery occlusion (90 and 120 minutes, respectively) with or without treatment with rapamycin at 30-minute poststroke. Changes in middle cerebral artery and collateral perfusion territories were measured by dual-site laser Doppler. Reactivity to rapamycin was studied using isolated and pressurized leptomeningeal anastomoses. Brain injury was measured histologically or with triphenyltetrazolium chloride staining.
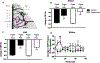
Results: In Wistar rats, rapamycin increased collateral perfusion (43±17%), increased reperfusion cerebral blood flow (16±8%) and significantly reduced infarct volume (35±6 versus 63±8 mm3, P<0.05). Rapamycin dilated leptomeningeal anastomoses by 80±9%, which was abolished by nitric oxide synthase inhibition. In spontaneously hypertensive rats, rapamycin increased collateral perfusion by 32±25%, reperfusion cerebral blood flow by 44±16%, without reducing acute infarct volume 2 hours postreperfusion. Reperfusion cerebral blood flow was a stronger predictor of brain damage than collateral perfusion in both Wistar and spontaneously hypertensive rats.
Conclusions: Rapamycin increased collateral perfusion and reperfusion cerebral blood flow in both Wistar and comorbid spontaneously hypertensive rats that appeared to be mediated by enhancing eNOS activation. These findings suggest that rapamycin may be an effective acute therapy for increasing collateral flow and as an adjunct therapy to thrombolysis or thrombectomy to improve reperfusion blood flow.
Keywords: dilation; hypertension; neuroprotection; reperfusion; stroke.
Conflict of interest statement
Disclosures
AMB is a senior medical science advisor and co-founder of Brainomix, a company that develops electronic ASPECTS (e-ASPECTS), an automated method to evaluate ASPECTS in stroke patients. All other authors declare no conflict of interest.
Figures




References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics-2019 update a report from the american heart association. Circulation. 2019;139:E56–E528 - PubMed
-
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

