Effect of nanostructured lipid carriers on transdermal delivery of tenoxicam in irradiated rats
- PMID: 32772730
- PMCID: PMC7470136
- DOI: 10.1080/10717544.2020.1803448
Effect of nanostructured lipid carriers on transdermal delivery of tenoxicam in irradiated rats
Abstract
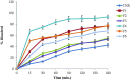
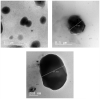
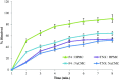
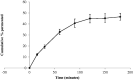
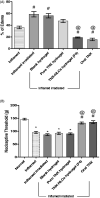
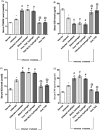
Transdermal delivery of non-steroidal anti-inflammatory drugs (NSAIDs) is an effective route of drug administration, as it directs the drug to the inflamed site with reduced incidence of systemic adverse effects such as gastric hemorrhage and ulcers. Tenoxicam (TNX) is a member of NSAIDs that are marketed only as oral tablets due to very poor absorption through the skin. The current study intended to formulate and characterize a hydrogel loaded with nanostructured lipid carriers (NLCs) to enhance the transdermal delivery of TNX. Six formulations of TNX were formulated by slight modifications of high shear homogenization and ultrasonication method. The selected formula was characterized for their particle size, polydispersity index (PDI), zeta potential, entrapment efficiency (EE), in-vitro drug release and ex-vivo skin permeation studies. Moreover, the effectiveness of the developed formula was studied in-vivo using carrageenan-induced paw edema and hyperalgesia model in irradiated rats. Formula F4 was chosen from six formulations, as the average diameter was 679.4 ± 51.3 nm, PDI value of about 0.02, zeta potential of -4.24 mV, EE of 92.36%, globules nanoparticles without aggregations and absence of interactions in the developed formula. Additionally, the in-vivo study showed the efficacy of formula F4 (TNX-NLCs hydrogel) equivalent to oral TNX in reducing the exaggerated inflammatory response induced by carrageenan after irradiation. In conclusion, the present findings suggest that TNX-NLCs hydrogel could be a potential transdermal drug delivery system alternative to the oral formulation for the treatment of various inflammatory conditions.
Keywords: Tenoxicam; carrageenan; irradiated rats; nanostructured lipid carriers; transdermal delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Abdellatif AAH, El-Telbany DFA, Zayed G, Al-Sawahli MM. (2019). Hydrogel containing PEG-coated fluconazole nanoparticles with enhanced solubility and antifungal activity. J Pharm Innov 14:112–22.
-
- AbdelSamie SM, Kamel AO, Sammour OA, Ibrahim SM. (2016). Terbinafine hydrochloride nanovesicular gel: in vitro characterization, ex vivo permeation and clinical investigation. Eur J Pharm Sci 88:91–100. - PubMed
-
- Aburahma MH, Badr-Eldin SM. (2014). Compritol 888 ATO: a multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert Opin Drug Deliv 11:1865–83. - PubMed
-
- Algandaby MM, Al-Sawahli MM, Ahmed OA, et al. (2016). Curcumin-Zein nanospheres improve liver targeting and antifibrotic activity of curcumin in carbon tetrachloride-induced mice liver fibrosis. J Biomed Nanotechnol 12:1746–57. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
