Cryo-EM structure of VASH1-SVBP bound to microtubules
- PMID: 32773040
- PMCID: PMC7449697
- DOI: 10.7554/eLife.58157
Cryo-EM structure of VASH1-SVBP bound to microtubules
Abstract
The dynamic tyrosination-detyrosination cycle of α-tubulin regulates microtubule functions. Perturbation of this cycle impairs mitosis, neural physiology, and cardiomyocyte contraction. The carboxypeptidases vasohibins 1 and 2 (VASH1 and VASH2), in complex with the small vasohibin-binding protein (SVBP), mediate α-tubulin detyrosination. These enzymes detyrosinate microtubules more efficiently than soluble αβ-tubulin heterodimers. The structural basis for this substrate preference is not understood. Using cryo-electron microscopy (cryo-EM), we have determined the structure of human VASH1-SVBP bound to microtubules. The acidic C-terminal tail of α-tubulin binds to a positively charged groove near the active site of VASH1. VASH1 forms multiple additional contacts with the globular domain of α-tubulin, including contacts with a second α-tubulin in an adjacent protofilament. Simultaneous engagement of two protofilaments by VASH1 can only occur within the microtubule lattice, but not with free αβ heterodimers. These lattice-specific interactions enable preferential detyrosination of microtubules by VASH1.
Keywords: E. coli; cryo-electron microscopy; detyrosination; microtubule; molecular biophysics; posttranslational modification; structural biology; vasohibin.
© 2020, Li et al.
Conflict of interest statement
FL, YL, XY, HG, ZS, XL, LR, HY No competing interests declared
Figures

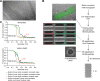






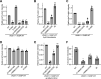
References
-
- Adamopoulos A, Landskron L, Heidebrecht T, Tsakou F, Bleijerveld OB, Altelaar M, Nieuwenhuis J, Celie PHN, Brummelkamp TR, Perrakis A. Crystal structure of the tubulin tyrosine carboxypeptidase complex VASH1-SVBP. Nature Structural & Molecular Biology. 2019;26:567–570. doi: 10.1038/s41594-019-0254-6. - DOI - PubMed
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Aillaud C, Bosc C, Peris L, Bosson A, Heemeryck P, Van Dijk J, Le Friec J, Boulan B, Vossier F, Sanman LE, Syed S, Amara N, Couté Y, Lafanechère L, Denarier E, Delphin C, Pelletier L, Humbert S, Bogyo M, Andrieux A, Rogowski K, Moutin MJ. Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science. 2017;358:1448–1453. doi: 10.1126/science.aao4165. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

