Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin
- PMID: 32773408
- PMCID: PMC7456234
- DOI: 10.1172/JCI134214
Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin
Abstract
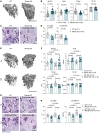
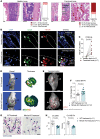
Although the control of bone-resorbing osteoclasts through osteocyte-derived RANKL is well defined, little is known about the regulation of osteoclasts by osteocyte death. Indeed, several skeletal diseases, such as bone fracture, osteonecrosis, and inflammation are characterized by excessive osteocyte death. Herein we show that osteoclasts sense damage-associated molecular patterns (DAMPs) released by necrotic osteocytes via macrophage-inducible C-type lectin (Mincle), which induced their differentiation and triggered bone loss. Osteoclasts showed robust Mincle expression upon exposure to necrotic osteocytes in vitro and in vivo. RNA sequencing and metabolic analyses demonstrated that Mincle activation triggers osteoclastogenesis via ITAM-based calcium signaling pathways, skewing osteoclast metabolism toward oxidative phosphorylation. Deletion of Mincle in vivo effectively blocked the activation of osteoclasts after induction of osteocyte death, improved fracture repair, and attenuated inflammation-mediated bone loss. Furthermore, in patients with osteonecrosis, Mincle was highly expressed at skeletal sites of osteocyte death and correlated with strong osteoclastic activity. Taken together, these data point to what we believe is a novel DAMP-mediated process that allows osteoclast activation and bone loss in the context of osteocyte death.
Keywords: Bone Biology; Bone disease; Immunology; Osteoclast/osteoblast biology; Osteoporosis.
Conflict of interest statement
Figures







Comment in
-
Osteocyte death promotes bone loss.Nat Rev Rheumatol. 2020 Oct;16(10):539. doi: 10.1038/s41584-020-0498-x. Nat Rev Rheumatol. 2020. PMID: 32843737 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

